Abstract
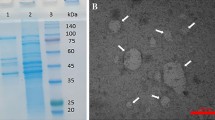
Akkermansia muciniphila (Am) shows a beneficial role as a probiotic in the treatment of metabolic syndrome. However, the mechanism remains to be elucidated. We tested the hypothesis that Am extracellular vesicles (AmEVs) have a protective effect against hypertension. Extracellular vesicles purified from anaerobically cultured Am were characterized by nanoparticle tracking analysis, transmission electron microscopy, and silver stain after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). AmEVs (1.0 × 1010 log particles/L) or vehicles were added into organ baths to induce vasorelaxation. In addition, AmEVs (1.0 × 108 or 1.0 × 109 particles/kg) or vehicles were injected into the tail veins of Wistar-Kyoto rats (WKYs) and spontaneously hypertensive rats (SHRs) weekly for 4 weeks. Peripheral blood mononuclear cells (PBMCs) and splenocytes isolated from both rat strains were analyzed by flow cytometry, RT-qPCR, and western blot. AmEVs affected neither vascular contraction nor endothelial relaxation in thoracic aortas. Moreover, AmEVs protected against the development of hypertension in SHRs without a serious adverse reaction. Additionally, AmEVs increased the population of T regulatory (Treg) cells and tended to reduce proinflammatory cytokines. These results indicate that AmEVs have a protective effect against hypertension without a serious adverse reaction. Therefore, it is foreseen that AmEVs may be utilized as a novel therapeutic for the treatment of hypertension.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133:2434–46.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013;110:9066–71.
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS one. 2013;8:e76520.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727.
Liu ZZ, Jose PA, Yang J, Zeng C. Importance of extracellular vesicles in hypertension. Exp Biol Med. 2021;246:342–53.
Jian H, Liu Y, Wang X, Dong X, Zou X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes? Int J Mol Sci. 2023;24:3900.
Xie J, Li Q, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022;40:1173–94.
Ashrafian F, Shahriary A, Behrouzi A, Moradi HR, Keshavarz Azizi Raftar S, Lari A, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155.
Pluznick JL. Microbial short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep. 2017;19:1–5.
Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206.
Ren H, Zhu B, An Y, Xie F, Wang Y, Tan Y. Immune communication between the intestinal microbiota and the cardiovascular system. Immunol Lett. 2023;254:13–20.
Zhang G, Lin X, Shao Y, Su C, Tao J, Liu X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin Exp Hypertens. 2020;42:257–65.
Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–93.
Bergmann J, Yamori Y, Okamoto K. Spontaneous hypertension in the rat: A model for human “essential” hypertension. Verh Dtsch Ges Inn Med. 1974;80:168–70.
Folkow B, Hallback M, Genest J, Koiw E, Kuchel O. Physiopathology of spontaneous hypertension in rats. Hypertension. 1977;507–29.
Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW, et al. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res. 2016;119:1101–15.
Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36:635–47.
Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76.
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450.
Diaz-Garrido N, Bonnin S, Riera M, Gimenez R, Badia J, Baldoma L. Transcriptomic microRNA Profiling of Dendritic Cells in Response to Gut Microbiota-Secreted Vesicles. Cells. 2020;9:1534.
Matthias J, Heink S, Picard F, Zeiträg J, Kolz A, Chao Y-Y, et al. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J Clin Investig. 2020;130:4587–600.
Taylor LE, Gillis EE, Musall JB, Baban B, Sullivan JC. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol. 2018;315:H1713–H23.
Almolda B, Costa M, Montoya M, Gonzalez B, Castellano B. Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat. PloS One. 2011;6:e27473.
Kim C-W, Kim JY, Lee S, Kim I. Dahl salt-resistant rats are protected against angiotensin II-induced hypertension. Biochem Pharmacol. 2022;203:115193.
Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22.
Katsuki M, Hirooka Y, Kishi T, Sunagawa K. Decreased proportion of Foxp3+ CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertens. 2015;33:773–83.
Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114:1547–63.
Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66.
Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3.
Bae M, Cassilly CD, Liu X, Park S-M, Tusi BK, Chen X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature. 2022;608:168–73.
Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63.
Itani H, McMaster W Jr, Saleh M, Nazarewicz R, Mikolajczyk T, Kaszuba A, et al. Activation of human T cells in hypertension novelty and significance. Hypertension. 2016;68:123–32.
Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93:489–97.
Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond G. IL‐1β and IL‐18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171:5589–602.
Kim JY, Lee E, Koo S, Kim CW, Kim I. Transfer of Th17 from adult spontaneous hypertensive rats accelerates development of hypertension in juvenile spontaneous hypertensive rats. BioMed Res Int. 2021;2021:1–13.
Kimura A, Kishimoto T. IL‐6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5.
Kim JY, Lee S, Jang S, Kim C-W, Gu B-H, Kim M, et al. T helper cell polarity determines salt sensitivity and hypertension development. Hypertens Res. 2023;46:2168–78.
Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517–32.
Zheng M, Han R, Yuan Y, Xing Y, Zhang W, Sun Z, et al. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Front Immunol. 2023;13:1089600.
Luo X, Han Z, Kong Q, Wang Y, Mou H, Duan X. Clostridium butyricum Prevents Dysbiosis and the Rise in Blood Pressure in Spontaneously Hypertensive Rats. Int J Mol Sci. 2023;24:4955.
Kim Y-K, Hyun-Taek P. Composition for treating or preventing metabolic disease, containing, as active ingredient, extracellular vesicles derived from akkermansia muciniphila bacteria. U.S. Patent Application No. 15/312,419.
Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PloS One. 2013;8:e71751.
Choi J, Kim Y-K, Han P-L. Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol. 2019;28:158.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2021R1A2B502001763, and 2021R1A4A1021617), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C0001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.Y., Kim, CW., Oh, S.Y. et al. Akkermansia muciniphila extracellular vesicles have a protective effect against hypertension. Hypertens Res (2024). https://doi.org/10.1038/s41440-024-01627-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41440-024-01627-5