Abstract
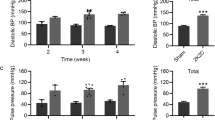
High salt intake induces hypertension and enhances stroke onset. However, whether an increase in brain sodium exposure itself is harmful and has poor prognosis remains unknown. Therefore, we employed hypertensive rats that underwent intracerebroventricular (ICV) infusion of sodium for 28 days and evaluated stroke onset and related cytotoxic brain injuries. Forty-seven spontaneously hypertensive stroke-prone (SHRSP) and 39 normotensive rats (Wistar Kyoto rats [WKY]) underwent persistent ICV infusion of the following four solutions: artificial cerebrospinal fluid, 0.9%, 2.7%, and 9% saline for 28 days. We evaluated stroke onset and all-cause mortality between SHRSP and WKY at each ICV sodium concentration as the primary endpoints. Our secondary objective was to explore histological brain injuries associated with SHRSP induced by high sodium ICV. The results indicated that ICV infusion of 2.7% and 9% sodium showed a significant increase in stroke onset, decrease in body weight, and increase rate of brain water content in SHRSP compared to WKY. Increased blood pressure was not observed for ICV infusion of high sodium, while serum sodium concentration was significantly increased in SHRSP compared to WKY. Histological evaluations revealed that higher sodium infusion significantly increased the number of activated microglia, superoxide, neuronal cell loss, and microbleeds compared to WKY and SHRSP with 0.9% sodium. We conclude that persistent exposure to high sodium in the brain is one of the risk factors for stroke onset upregulating cerebral microbleeds and oxidative stress in hypertensive rats.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–14.
Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 2015;65:1042–50.
Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100:519–24.
Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21:240–9.
Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–53.
Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55.
Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9.
Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64.
Nakagawa T, Hasegawa Y, Uekawa K, Ma M, Katayama T, Sueta D, et al. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2:e000375.
Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68.
Smykiewicz P, Segiet A, Keag M, Żera T. Proinflammatory cytokines and ageing of the cardiovascular-renal system. Mech Ageing Dev. 2018;175:35–45.
Subha M, Pal P, Pal GK, Habeebullah S, Adithan C, Sridhar MG. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, low-grade inflammation, and oxidative stress in pregnancy-induced hypertension. Clin Exp Hypertens. 2016;38:666–72.
Hasegawa Y, Takemoto Y, Hayashi K, Kameno K, Kim-Mitsuyama S. The endogenous and exogenous brain-derived neurotrophic factor plays pivotal roles in the pathogenesis of stroke onset in high salt-loaded hypertensive rats. Exp Gerontol. 2021;147:111286.
Takane K, Hasegawa Y, Lin B, Koibuchi N, Cao C, Yokoo T, et al. Detrimental effects of centrally administered angiotensin II are enhanced in a mouse model of alzheimer disease independently of blood pressure. J Am Heart Assoc. 2017;6:e004897.
Takemoto Y, Hasegawa Y, Hayashi K, Cao C, Hamasaki T, Kawano T, et al. The stabilization of central sympathetic nerve activation by renal denervation prevents cerebral vasospasm after subarachnoid hemorrhage in rats. Transl Stroke Res. 2020;11:528–40.
Hasegawa Y, Nakagawa T, Uekawa K, Ma M, Lin B, Kusaka H, et al. Therapy with the combination of amlodipine and irbesartan has persistent preventative effects on stroke onset associated with BDNF preservation on cerebral vessels in hypertensive rats. Transl Stroke Res. 2016;7:79–87.
Kubota Y, Umegaki K, Kagota S, Tanaka N, Nakamura K, Kunitomo M, et al. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol Pharm Bull. 2006;29:1756–8.
Hayashi K, Hasegawa Y, Takemoto Y, Cao C, Takeya H, Komohara Y, et al. Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer’s disease. Exp Gerontol. 2019;120:1–5.
Ma M, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Nakagawa T, et al. DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc Diabetol. 2015;14:54.
Smolek T, Cubinkova V, Brezovakova V, Valachova B, Szalay P, Zilka N, et al. Genetic background influences the propagation of tau pathology in transgenic rodent models of tauopathy. Front Aging Neurosci. 2019;11:343.
Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4.
Young K, Morrison H. Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J Vis Exp. 2018:57648. https://doi.org/10.3791/57648.
Morrison H, Young K, Qureshi M, Rowe RK, Lifshitz J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep. 2017;7:13211.
Lin B, Hasegawa Y, Takane K, Koibuchi N, Cao C, Kim-Mitsuyama S. High-fat-diet intake enhances cerebral amyloid angiopathy and cognitive impairment in a mouse model of Alzheimer’s disease, independently of metabolic disorders. J Am Heart Assoc. 2016;5:e003154.
Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, et al. Critical role of angiotensin II in excess salt-induced brain oxidative stress of stroke-prone spontaneously hypertensive rats. Stroke. 2005;36:1083–8.
Andersson B. Thirst–and brain control of water balance. Am Sci. 1971;59:408–15.
Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, et al. Na(+)] increases in body fluids sensed by central Na(x) induce sympathetically mediated blood pressure elevations via H(+)-dependent activation of ASIC1a. Neuron. 2019;101:60–75.e66.
Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–43.
Koibuchi N, Hasegawa Y, Katayama T, Toyama K, Uekawa K, Sueta D, et al. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc Diabetol. 2014;13:157.
Li QQ, Li JY, Zhou M, Qin ZH, Sheng R. Targeting neuroinflammation to treat cerebral ischemia – the role of TIGAR/NADPH axis. Neurochem Int. 2021;148:105081.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by research grants from The Salt Science Research Foundation (2034), JSPS KAKENHI (21K09193), and IUHW Research Grants.
Author information
Authors and Affiliations
Contributions
SK and YH contributed to study conception and design. SK, KF, ST, KK, and HU performed the experiments. YH performed the statistical analysis. MM helped with the interpretations. SK wrote the first draft of the manuscript. YH revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving animals were conducted in accordance with the ethical standards of the institution or practice at which the studies conducted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kajiwara, S., Hasegawa, Y., Fujimori, K. et al. Persistent brain exposure to high sodium induces stroke onset by upregulation of cerebral microbleeds and oxidative stress in hypertensive rats. Hypertens Res 47, 78–87 (2024). https://doi.org/10.1038/s41440-023-01447-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01447-z