Abstract
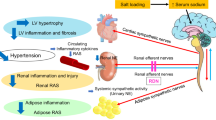
Adrenal gland hormones play a critical role in the development and maintenance of hypertension. Adrenal ablation has been used to treat primary aldosteronism but not essential hypertension. The present study aimed to investigate the effectiveness and safety of unilateral adrenal gland ablation in treating spontaneously hypertensive rats (SHR). SHR and Wistar-Kyoto rats (WKY) were subjected to unilateral adrenal ablation with injection of anhydrous ethanol or a sham procedure. Blood pressure was monitored by both tail-cuff plethysmography and telemetry until 6 months after the procedure. Adrenal ablation significantly lowered systolic and diastolic blood pressure of the SHR (P < 0.05 or P < 0.01) but not WKY from 4 to 24 weeks after the procedure. Adrenal ablation substantially damaged adrenal cortex and medulla with fibrosis in SHR and WKY rats. The ablation procedure remarkably reduced the levels of renin, angiotensin II, aldosterone, cortisol, noradrenaline, and epinephrine in SHR (all P < 0.05) but not in WKY. Hypokalemia in SHR was significantly improved by adrenal ablation (P < 0.05), while the serum sodium levels were not affected by adrenal ablation in either SHR or WKY rats. Additionally, adrenal ablation improved cardiac, renal, and vascular remodeling and function measured 3 months after the procedure in SHR. In conclusion, the present study shows that ethanol ablation of adrenal gland can effectively lower blood pressure and prevent target organ damage in SHR. These findings suggest that unilateral debulking of the adrenal gland using ethanol ablation is a promising therapeutic strategy for treating hypertension.

Methods: Spontaneously hypertensive rats (SHR) and Wistar-Kyoto rats (WKY) were subjected to unilateral adrenal ablation with injection of ethanol or a sham procedure. Blood pressure was monitored for 24 weeks. Results: Adrenal ablation significantly lowered systolic and diastolic blood pressure of SHR but not WKY from 4 to 24 weeks after the procedure. Conclusion: Unilateral debulking of the adrenal gland using ethanol ablation is a promising therapeutic strategy for treating hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Liu D, Wang J, Hu H, Gu G, Ding R, Xie R, et al. The effects of renal nerve denervation on blood pressure and target organs in different hypertensive rat models. Int J Hypertens. 2021;2021:8615253.
Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90.
Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension. 2020;76:113–20.
Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res. 2019;124:1061–70.
Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75.
Gorini S, Kim SK, Infante M, Mammi C, La Vignera S, Fabbri A, et al. Role of aldosterone and mineralocorticoid receptor in cardiovascular aging. Front Endocrinol (Lausanne). 2019;10:584.
Vorselaars W, Nell S, Postma EL, Zarnegar R, Drake FT, Duh QY, et al. Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surg. 2019;154:e185842.
Suurd DPD, Vorselaars W, Van Beek DJ, Borel Rinkes IHM, Spiering W, Valk GD, et al. Assessing outcomes after adrenalectomy for primary aldosteronism - early is accurate: retrospective cohort study. Ann Surg. 2022;276:929–34.
Katabami T, Fukuda H, Tsukiyama H, Tanaka Y, Takeda Y, Kurihara I, et al. Clinical and biochemical outcomes after adrenalectomy and medical treatment in patients with unilateral primary aldosteronism. J Hypertens. 2019;37:1513–20.
Szabo Yamashita T, Shariq OA, Foster TR, Lyden ML, Dy BM, Young WF Jr., et al. Unilateral adrenalectomy for primary aldosteronism due to bilateral adrenal disease can result in resolution of hypokalemia and amelioration of hypertension. World J Surg. 2023;47:314–8.
Zhou Y, Liu Q, Wang X, Wan J, Liu S, Luo T, et al. Adrenal ablation versus mineralocorticoid receptor antagonism for the treatment of primary aldosteronism: a single-center prospective cohort study. Am J Hypertens. 2022;35:1014–23.
Dong H, Zou Y, He J, Deng Y, Chen Y, Song L, et al. Superselective adrenal arterial embolization for idiopathic hyperaldosteronism: 12-month results from a proof-of-principle trial. Catheter Cardiovasc Inter. 2021;97:976–81.
Zhao Z, Liu X, Zhang H, Li Q, He H, Yan Z, et al. Catheter-based adrenal ablation remits primary aldosteronism: a randomized medication-controlled trial. Circulation. 2021;144:580–2.
Qiu J, Li N, Xiong HL, Yang J, Li YD, Hu CK, et al. Superselective adrenal arterial embolization for primary aldosteronism without lateralized aldosterone secretion: an efficacy and safety, proof-of-principle study. Hypertens Res. 2023;46:14.
Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–64.
Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, et al. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H555–64.
Wang X, Wang J, Luo H, Chen C, Pei F, Cai Y, et al. Prenatal lipopolysaccharide exposure causes mesenteric vascular dysfunction through the nitric oxide and cyclic guanosine monophosphate pathway in offspring. Free Radic Biol Med. 2015;86:322–30.
Wang M, Han W, Zhang M, Fang W, Zhai X, Guan S, et al. Long-term renal sympathetic denervation ameliorates renal fibrosis and delays the onset of hypertension in spontaneously hypertensive rats. Am J Transl Res. 2018;10:4042–53.
Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517–32.
Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cifkova R, Dominiczak AF, et al. Hypertension. Nat Rev Dis Prim. 2018;4:18014.
Bechmann N, Berger I, Bornstein SR, Steenblock C. Adrenal medulla development and medullary-cortical interactions. Mol Cell Endocrinol. 2021;528:111258.
Berger I, Werdermann M, Bornstein SR, Steenblock C. The adrenal gland in stress—adaptation on a cellular level. J Steroid Biochem Mol Biol. 2019;190:198–206.
Berends AMA, Eisenhofer G, Fishbein L, Horst-Schrivers A, Kema IP, Links TP, et al. Intricacies of the molecular machinery of catecholamine biosynthesis and secretion by chromaffin cells of the normal adrenal medulla and in pheochromocytoma and paraganglioma. Cancers (Basel). 2019;11:1121.
Wexler BC, McMurtry JP. Differences in adrenal cholesterol, ascorbic acid, circulating corticosterone and aldosterone during the onset of hypertension in SHR vs WKy rats. Cardiovasc Res. 1982;16:573–9.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68.
Maron BA, Leopold JA. Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010;121:934–9.
Freeman MW, Halvorsen YD, Marshall W, Pater M, Isaacsohn J, Pearce C, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405.
Leopold JA, Ingelfinger JR. Aldosterone and treatment-resistant hypertension. N Engl J Med. 2023;388:464–7.
Azizi M. Decreasing the effects of aldosterone in resistant hypertension—a success story. N Engl J Med. 2023;388:461–3.
Heinrich DA, Adolf C, Holler F, Lechner B, Schneider H, Riester A, et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab. 2019;104:5658–64.
Wang X, Heinrich DA, Kunz SL, Heger N, Sturm L, Uhl O, et al. Characteristics of preoperative steroid profiles and glucose metabolism in patients with primary aldosteronism developing adrenal insufficiency after adrenalectomy. Sci Rep. 2021;11:11181.
Zhang H, Li Q, Liu X, Zhao Z, He H, Sun F, et al. Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: an efficacy and safety, proof-of-principle trial. J Clin Hypertens (Greenwich). 2020;22:1618–26.
Zhou Y, Wang D, Liu Q, Hou J, Wang P. Case report: percutaneous adrenal arterial embolization cures resistant hypertension. Front Cardiovasc Med. 2022;9:1013426.
Jama HA, Muralitharan RR, Xu C, O’Donnell JA, Bertagnolli M, Broughton BRS, et al. Rodent models of hypertension. Br J Pharm. 2022;179:918–37.
Kenyon CJ, DeConti GA, Cupolo NA, Morris DJ. The role of aldosterone in the development of hypertension in spontaneously hypertensive rats. Endocrinology. 1981;109:1841–5.
Stern N, Beck FJ, Eggena P, Phillips D, Sowers JR. Corticosteroid modulation of the renin system and blood pressure in the spontaneously hypertensive rat. Clin Exp Hypertens A. 1983;5:1543–57.
Bucher B, Stoclet JC. Adrenomedullary and beta-adrenergic participation in enhanced sympathetic pressor responses of spontaneously hypertensive rats. Eur J Pharm. 1987;138:233–8.
Chun TY, Chander PN, Kim JW, Pratt JH, Stier CT Jr. Aldosterone, but not angiotensin II, increases profibrotic factors in kidney of adrenalectomized stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2008;295:E305–12.
Chander PN, Rocha R, Ranaudo J, Singh G, Zuckerman A, Stier CT Jr. Aldosterone plays a pivotal role in the pathogenesis of thrombotic microangiopathy in SHRSP. J Am Soc Nephrol. 2003;14:1990–7.
Kokubo M, Uemura A, Matsubara T, Murohara T. Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens Res. 2005;28:601–9.
Sen S, Smeby RR, Bumpus FM. Renin in rats with spontaneous hypertension. Circ Res. 1972;31:876–80.
Funding
This work was partially supported by a grant from National Natural Science Foundation of China (81970262 to PW) and a grant from the Foundation of Chengdu Medical College (CYZYB22-01 to XW). The funding source had no role in the design of the study and did not participate in study execution, analysis or interpretation of the data, or the decision to submit the results for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Luo, T., Yang, Y. et al. Unilateral chemical ablation of the adrenal gland lowers blood pressure and alleviates target organ damage in spontaneously hypertensive rats. Hypertens Res 46, 2693–2704 (2023). https://doi.org/10.1038/s41440-023-01444-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01444-2
Keywords
This article is cited by
-
Lowering of blood pressure by chemical ablation of the unilateral adrenal gland in spontaneously hypertensive rats
Hypertension Research (2023)