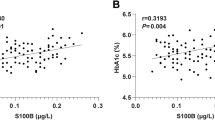
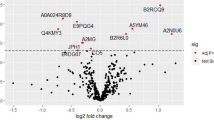
Abstract
Hypertensive disorders of pregnancy (HDP) result in major maternal and fetal complications. Our study aimed to find a panel of protein markers to identify HDP by applying machine-learning models. The study was conducted on a total of 133 samples, divided into four groups, healthy pregnancy (HP, n = 42), gestational hypertension (GH, n = 67), preeclampsia (PE, n = 9), and ante-partum eclampsia (APE, n = 15). Thirty circulatory protein markers were measured using Luminex multiplex immunoassay and ELISA. Significant markers were screened for potential predictive markers by both statistical and machine-learning approaches. Statistical analysis found seven markers such as sFlt-1, PlGF, endothelin-1(ET-1), basic-FGF, IL-4, eotaxin and RANTES to be altered significantly in disease groups compared to healthy pregnant. Support vector machine (SVM) learning model classified GH and HP with 11 markers (eotaxin, GM-CSF, IL-4, IL-6, IL-13, MCP-1, MIP-1α, MIP-1β, RANTES, ET-1, sFlt-1) and HDP with 13 markers (eotaxin, G-CSF, GM-CSF, IFN-gamma, IL-4, IL-5, IL-6, IL-13, MCP-1, MIP-1β, RANTES, ET-1, sFlt-1). While logistic regression (LR) model classified PE with 13 markers (basic FGF, IL-1β, IL-1ra, IL-7, IL-9, MIP-1β, RANTES, TNF-alpha, nitric oxide, superoxide dismutase, ET-1, PlGF, sFlt-1) and APE by 12 markers (eotaxin, basic-FGF, G-CSF, GM-CSF, IL-1β, IL-5, IL-8, IL-13, IL-17, PDGF-BB, RANTES, PlGF). These markers may be used to diagnose the progression of healthy pregnant to a hypertensive state. Future longitudinal studies with large number of samples are needed to validate these findings.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, et al. Hypertensive disorders of pregnancy. J Prenat Med. 2009;3:1–5.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. https://doi.org/10.1161/HYPERTENSIONAHA.117.10803.
McElwain CJ, Tuboly E, McCarthy FP, McCarthy CM. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front Endocrinol. 2020;11:655.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–92. https://doi.org/10.1016/s0029-7844(03)00475-7.
Kyozuka H, Fujimori K, Hosoya M, Yasumura S, Yokoyama T, Sato A, et al. The Japan Environment and Children’s Study (JECS) in Fukushima Prefecture: pregnancy outcome after the Great East Japan Earthquake. Tohoku J Exp Med. 2018;246:27–33. https://doi.org/10.1620/tjem.246.27.
ACOG. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. https://doi.org/10.1097/AOG.0000000000003018.
Stefańska K, Zieliński M, Jankowiak M, Zamkowska D, Sakowska J, Adamski P, et al. Cytokine imprint in preeclampsia. Front Immunol. 2021;12:667841. https://doi.org/10.3389/fimmu.2021.667841.
Deng C, Ji X, Rainey C, Zhang J, Lu W. Integrating machine learning with human knowledge. iScience. 2020;23:101656 https://doi.org/10.1016/j.isci.2020.101656.
Sarker IH. Machine learning: algorithms, real-world applications and research directions. SN Comput Sci. 2021;2:160. https://doi.org/10.1007/s42979-021-00592-x.
Varghese B, Jala A, Meka S, Adla D, Jangili S, Talukdar RK, et al. Integrated metabolomics and machine learning approach to predict hypertensive disorders of pregnancy. Am J Obstet Gynecol MFM. 2022;5:100829. https://doi.org/10.1016/j.ajogmf.2022.100829.
Redman CWG, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–43. https://doi.org/10.1111/j.1600-0897.2010.00831.x.
Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. https://doi.org/10.1016/s0143-4004(83)80043-5.
Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. https://doi.org/10.1016/0002-9378(89)90665-0.
Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. https://doi.org/10.1146/annurev-pathol-121808-102149.
Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397–412. https://doi.org/10.1016/j.immuni.2018.07.017.
Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013;69:545–57. https://doi.org/10.1111/aji.12088.
Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–9. https://doi.org/10.1086/522182.
Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16:13442–73. https://doi.org/10.3390/ijms160613442.
Adela R, Borkar RM, Mishra N, Bhandi MM, Vishwakarma G, Varma BA, et al. Lower serum vitamin D metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Front Immunol. 2017;8:273. https://doi.org/10.3389/fimmu.2017.00273.
Yarim GF, Karahan S, Nisbet C. Elevated plasma levels of interleukin 1 beta, tumour necrosis factor alpha and monocyte chemotactic protein 1 are associated with pregnancy toxaemia in ewes. Vet Res Commun. 2007;31:565–73. https://doi.org/10.1007/s11259-007-3551-1.
Mellembakken JR, Solum NO, Ueland T, Videm V, Aukrust P. Increased concentrations of soluble CD40 ligand, RANTES and GRO-alpha in preeclampsia–possible role of platelet activation. Thromb Haemost. 2001;86:1272–6.
Kauma S, Takacs P, Scordalakes C, Walsh S, Green K, Peng T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol. 2002;100:706–14. https://doi.org/10.1016/s0029-7844(02)02169-5.
Hentschke MR, Krauspenhar B, Guwzinski A, Caruso FB, Silveira ID, Antonello IC, et al. PP040. Expression of RANTES (CCL5) in maternal plasma, fetal plasma and placenta in pre-eclampsia and normotensive controls. Pregnancy Hypertens. 2012;2:263. https://doi.org/10.1016/j.preghy.2012.04.151.
Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–9. https://doi.org/10.1038/325257a0.
Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–13. https://doi.org/10.1161/01.res.68.1.106.
Lindner V, Majack RA, Reidy MA. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990;85:2004–8. https://doi.org/10.1172/JCI114665.
Krishnamurthy P, Bird IM, Sheppard C, Magness RR. Effects of angiogenic growth factors on endothelium-derived prostacyclin production by ovine uterine and placental arteries. Prostaglandins Other Lipid Mediat. 1999;57:1–12. https://doi.org/10.1016/s0090-6980(98)00066-5.
Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–75. https://doi.org/10.1016/s0002-9378(98)70160-7.
Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–51. https://doi.org/10.1016/0140-6736(93)90889-o.
Kurz C, Hefler L, Zeisler H, Schatten C, Husslein P, Tempfer C. Maternal basic fibroblast growth factor serum levels are associated with pregnancy-induced hypertension. J Soc Gynecol Investig. 2001;8:24–6.
Hohlagschwandtner M, Knöfler M, Ploner M, Zeisler H, Joura EA, Husslein P. Basic fibroblast growth factor and hypertensive disorders in pregnancy. Hypertens Pregnancy. 2002;21:235–41. https://doi.org/10.1081/PRG-120016790.
Ozkan S, Vural B, Filiz S, Coştur P, Dalçik H. Placental expression of insulin-like growth factor-I, fibroblast growth factor-basic, and neural cell adhesion molecule in preeclampsia. J Matern Fetal Neonatal Med. 2008;21:831–8. https://doi.org/10.1080/14767050802251024.
Spence T, Allsopp PJ, Yeates AJ, Mulhern MS, Strain JJ, McSorley EM. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J Pregnancy. 2021;2021:6649608. https://doi.org/10.1155/2021/6649608.
Kang L, Chen C-H, Yu C-H, Chang CH, Chang FM. An association study of interleukin-4 gene and preeclampsia in Taiwan. Taiwan J Obstet Gynecol. 2014;53:215–9. https://doi.org/10.1016/j.tjog.2014.04.017.
Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma IL-4, IL-8, IL-12, interferon-γ and CRP levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birth weight. J Matern Fetal Neonatal Med. 2012;25:1569–73. https://doi.org/10.3109/14767058.2011.648233.
Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, et al. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 2015;28:135–42. https://doi.org/10.1093/ajh/hpu100.
Chatterjee P, Kopriva SE, Chiasson VL, Young KJ, Tobin RP, Newell-Rogers K, et al. Interleukin-4 deficiency induces mild preeclampsia in mice. J Hypertens. 2013;31:1414–23. https://doi.org/10.1097/HJH.0b013e328360ae6c.
Cottrell JN, Amaral LM, Harmon A, Cornelius DC, Cunningham MW, Vaka VR, et al. Interleukin-4 supplementation improves the pathophysiology of hypertension in response to placental ischemia in RUPP rats. Am J Physiol Regul Integr Comp Physiol. 2019;316:R165–71. https://doi.org/10.1152/ajpregu.00167.2018.
Chau SE, Murthi P, Wong MH, Whitley GS, Brennecke SP, Keogh RJ. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum Reprod. 2013;28:1497–507. https://doi.org/10.1093/humrep/det060.
Du M, Basu A, Fu D, Wu M, Centola M, Jenkins AJ, et al. Serum inflammatory markers and preeclampsia in type 1 diabetes: a prospective study. Diabetes Care. 2013;36:2054–61. https://doi.org/10.2337/dc12-1934.
Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. https://doi.org/10.1016/j.jri.2005.10.007.
Khetsuriani T, Chabashvili N, Sanikidze T. Role of endothelin-1 and nitric oxide level in pathogenesis preeclampsia. Georgian Med News. 2006;141:17–21.
George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–9. https://doi.org/10.1038/AJH.2011.99.
Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–41. https://doi.org/10.1038/jhh.2011.29.
Lu Y-P, Hasan AA, Zeng S, Hocher B. Plasma ET-1 concentrations are elevated in pregnant women with hypertension -meta-analysis of clinical studies. Kidney Blood Press Res. 2017;42:654–63. https://doi.org/10.1159/000482004.
Verdonk K, Saleh L, Lankhorst S, Smilde JE, van Ingen MM, Garrelds IM, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65:1316–23. https://doi.org/10.1161/HYPERTENSIONAHA.115.05267.
Saleh L, Verdonk K, Visser W, van den Meiracker AH, Danser AH. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis. 2016;10:282–93. https://doi.org/10.1177/1753944715624853.
Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–7. https://doi.org/10.1210/jcem-71-6-1675.
Eddy AC, Bidwell GL, George EM. Pro-angiogenic therapeutics for preeclampsia. Biol Sex Differ. 2018;9:36. https://doi.org/10.1186/s13293-018-0195-5.
Tripathi R, Ralhan R, Saxena S, Salhan S, Rath G. Soluble VEGFR-1 in pathophysiology of pregnancies complicated by hypertensive disorders: the Indian scenario. J Hum Hypertens. 2013;27:107–14. https://doi.org/10.1038/jhh.2012.2.
Tang Y, Ye W, Liu X, Lv Y, Yao C, Wei J. VEGF and sFLT-1 in serum of PIH patients and effects on the foetus. Exp Ther Med. 2019;17:2123–8. https://doi.org/10.3892/etm.2019.7184.
Amosco MD, Villar VA, Naniong JM, David-Bustamante LM, Jose PA, Palmes-Saloma CP. VEGF-A and VEGFR1 SNPs associate with preeclampsia in a Philippine population. Clin Exp Hypertens. 2016;38:578–85. https://doi.org/10.3109/10641963.2016.1174252.
Herraiz I, Llurba E, Verlohren S, Galindo A, Spanish Group for the Study of Angiogenic Markers in P. Update on the diagnosis and prognosis of preeclampsia with the aid of the sFlt-1/ PlGF ratio in singleton pregnancies. Fetal Diagn Ther. 2018;43:81–9. https://doi.org/10.1159/000477903.
Ding G, Liping L, Moli D, Wuliyeti A, Shaohe Z, Huijuan W, et al. A study of the association between the sFlt-1/PIGF ratio and preeclampsia in Xinjiang Uygur Autonomous Region of China. Artif Cells Nanomed Biotechnol. 2018;46:S281–6. https://doi.org/10.1080/21691401.2018.1491480.
Karge A, Seiler A, Flechsenhar S, Haller B, Ortiz JU, Lobmaier SM, et al. Prediction of adverse perinatal outcome and the mean time until delivery in twin pregnancies with suspected pre-eclampsia using sFlt-1/PIGF ratio. Pregnancy Hypertens. 2021;24:37–43. https://doi.org/10.1016/j.preghy.2021.02.003.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161.e1–161.e11. https://doi.org/10.1016/j.ajog.2009.09.016.
Maynard SE, Min J-Y, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. https://doi.org/10.1172/JCI17189.
Mazer Zumaeta A, Wright A, Syngelaki A, Maritsa VA, Da Silva AB, Nicolaides KH. Screening for pre-eclampsia at 11-13 weeks’ gestation: use of pregnancy-associated plasma protein-A, placental growth factor or both. Ultrasound Obstet Gynecol J Int Soc Ultrasound Obstet Gynecol. 2020;56:400–7. https://doi.org/10.1002/uog.22093.
Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. https://doi.org/10.1084/jem.184.2.539.
Sonek J, Krantz D, Carmichael J, Downing C, Jessup K, Haidar Z, et al. First-trimester screening for early and late preeclampsia using maternal characteristics, biomarkers, and estimated placental volume. Am J Obstet Gynecol. 2018;218:126.e1–126.e13. https://doi.org/10.1016/j.ajog.2017.10.024.
Venny 2.1.0. https://bioinfogp.cnb.csic.es/tools/venny/index.html. Accessed 21 Apr 2023.
Acknowledgements
The authors sincerely acknowledge the Department of Pharmaceuticals (DoP) under the Ministry of Chemicals and Fertilizers, Government of India, and Dr. USN Murty, Director, NIPER-G for their extensive support.
Funding
This study is funded by the Institutional Core Grant, NIPER-Guwahati, Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: RA, and BV. Statistical and Bioinformatic analysis: JVNJ, SJ, SRM, BV and RA. Clinical study: BV, CAJ, RA and RKT. Draft manuscript preparation: BV, RA, JVNJ, SJ, and SRM. Final manuscript preparation: BV and RA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Varghese, B., Joy, C.A., Josyula, J.V.N. et al. Machine learning-based protein signatures for differentiating hypertensive disorders of pregnancy. Hypertens Res 46, 2513–2526 (2023). https://doi.org/10.1038/s41440-023-01348-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01348-1
Keywords
This article is cited by
-
The association of a panel of circulating markers with hypertensive disorders of pregnancy: the jury is still out
Hypertension Research (2023)
-
Optimal blood pressure and improvement of achievement rate
Hypertension Research (2023)