Abstract
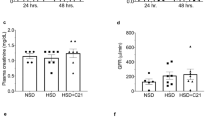
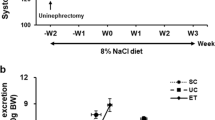
Salt-sensitive hypertension is associated with poor clinical outcomes. The epithelial sodium channel (ENaC) in the kidney plays pivotal roles in sodium reabsorption and blood pressure regulation, in which its γ subunit is activated by extracellular serine proteases. In proteinuric nephropathies, plasmin filtered through injured glomeruli reportedly activates γENaC in the distal nephron and causes podocyte injury. We previously reported that Dahl salt-sensitive (DS) rats fed a high-salt (HS) diet developed hypertension and proteinuria along with γENaC activation and that a synthetic serine protease inhibitor, camostat mesilate, mitigated these changes. However, the role of plasmin in DS rats remained unclear. In this study, we evaluated the relationship between plasmin and hypertension as well as podocyte injury and the effects of plasmin inhibitors in DS rats. Five-week-old DS rats were divided into normal-salt diet, HS diet, and HS+plasmin inhibitor (either tranexamic acid [TA] or synthetic plasmin inhibitor YO-2) groups. After blood pressure measurement and 24 h urine collection over 5 weeks, rats were sacrificed for biochemical analyses. The HS group displayed severe hypertension and proteinuria together with activation of plasmin in urine and γENaC in the kidney, which was significantly attenuated by YO-2 but not TA. YO-2 inhibited the attachment of plasmin(ogen) to podocytes and alleviated podocyte injury by inhibiting apoptosis and inflammatory/profibrotic cytokines. YO-2 also suppressed upregulation of protease-activated receptor-1 and phosphorylated ERK1/2. These results indicate an important role of plasmin in the development of salt-sensitive hypertension and related podocyte injury, suggesting plasmin inhibition as a potential therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7.
Marunaka Y. Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Jpn J Physiol. 1997;47:499–511.
Mutchler SM, Kirabo A, Kleyman TR. Epithelial Sodium Channel and Salt-Sensitive Hypertension. Hypertension. 2021;77:759–67.
Oh YS, Warnock DG. Disorders of the epithelial Na(+) channel in Liddle’s syndrome and autosomal recessive pseudohypoaldosteronism type 1. Exp Nephrol. 2000;8:320–5.
Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–10.
Anand D, Hummler E, Rickman OJ. ENaC activation by proteases. Acta Physiol. 2022;235:e13811.
Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23.
Kitamura K, Tomita K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin Exp Nephrol. 2012;16:44–48.
Olivieri O, Chiecchi L, Pizzolo F, Castagna A, Raffaelli R, Gunasekaran M, et al. Urinary prostasin in normotensive individuals: correlation with the aldosterone to renin ratio and urinary sodium. Hypertens Res. 2013;36:528–33.
Uchimura K, Kakizoe Y, Onoue T, Hayata M, Morinaga J, Yamazoe R, et al. In vivo contribution of serine proteases to the proteolytic activation of gammaENaC in aldosterone-infused rats. Am J Physiol Ren Physiol. 2012;303:F939–943.
Artunc F, Wörn M, Schork A, Bohnert BN. Proteasuria-The impact of active urinary proteases on sodium retention in nephrotic syndrome. Acta Physiol. 2019;225:e13249.
Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310.
Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–91.
Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T, et al. Aberrant ENaC activation in Dahl salt-sensitive rats. J Hypertens. 2009;27:1679–89.
Maekawa A, Kakizoe Y, Miyoshi T, Wakida N, Ko T, Shiraishi N, et al. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. J Hypertens. 2009;27:181–9.
Raij L, Tian R, Wong JS, He JC, Campbell KN. Podocyte injury: the role of proteinuria, urinary plasminogen, and oxidative stress. Am J Physiol Ren Physiol. 2016;311:F1308–F1317.
Egerman MA, Wong JS, Runxia T, Mosoyan G, Chauhan K, Reyes-Bahamonde J, et al. Plasminogenuria is associated with podocyte injury, edema, and kidney dysfunction in incident glomerular disease. FASEB J. 2020;34:16191–204.
Kakizoe Y, Miyasato Y, Onoue T, Nakagawa T, Hayata M, Uchimura K, et al. A serine protease inhibitor attenuates aldosterone-induced kidney injuries via the suppression of plasmin activity. J Pharm Sci. 2016;132:145–53.
Mizumoto T, Kakizoe Y, Nakagawa T, Iwata Y, Miyasato Y, Uchimura K, et al. A serine protease inhibitor camostat mesilate prevents podocyte apoptosis and attenuates podocyte injury in metabolic syndrome model rats. J Pharm Sci. 2021;146:192–9.
Tamura Y, Hirado M, Okamura K, Minato Y, Fujii S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r-, and C1 esterase. Biochim Biophys Acta. 1977;484:417–22.
Nakagawa T, Kakizoe Y, Iwata Y, Miyasato Y, Mizumoto T, Adachi M, et al. Doxycycline attenuates cisplatin-induced acute kidney injury through pleiotropic effects. Am J Physiol Ren Physiol. 2018;315:F1347–f1357.
Zhang G, Kernan KA, Collins SJ, Cai X, López-Guisa JM, Degen JL, et al. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol. 2007;18:846–59.
Sharma R, Waller AP, Agrawal S, Wolfgang KJ, Luu H, Shahzad K, et al. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J Am Soc Nephrol. 2017;28:2618–30.
Law RHP, Wu G, Leung EWW, Hidaka K, Quek AJ, Caradoc-Davies TT, et al. X-ray crystal structure of plasmin with tranexamic acid-derived active site inhibitors. Blood Adv. 2017;1:766–71.
Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003;63:1417–33.
Okada Y, Tsuda Y, Tada M, Wanaka K, Okamoto U, Hijikata-Okunomiya A, et al. Development of potent and selective plasmin and plasma kallikrein inhibitors and studies on the structure-activity relationship. Chem Pharm Bull. 2000;48:1964–72.
Bohnert BN, Daiminger S, Wörn M, Sure F, Staudner T, Ilyaskin AV, et al. Urokinase-type plasminogen activator (uPA) is not essential for epithelial sodium channel (ENaC)-mediated sodium retention in experimental nephrotic syndrome. Acta Physiol. 2019;227:e13286.
Xiao M, Bohnert BN, Aypek H, Kretz O, Grahammer F, Aukschun U, et al. Plasminogen deficiency does not prevent sodium retention in a genetic mouse model of experimental nephrotic syndrome. Acta Physiol. 2021;231:e13512.
Hinrichs GR, Weyer K, Friis UG, Svenningsen P, Lund IK, Nielsen R, et al. Urokinase-type plasminogen activator contributes to amiloride-sensitive sodium retention in nephrotic range glomerular proteinuria in mice. Acta Physiol. 2019;227:e13362.
Andersen H, Hansen MH, Buhl KB, Stæhr M, Friis UG, Enggaard C, et al. Plasminogen Deficiency and Amiloride Mitigate Angiotensin II-Induced Hypertension in Type 1 Diabetic Mice Suggesting Effects Through the Epithelial Sodium Channel. J Am Heart Assoc. 2020;9:e016387.
Andersen H, Friis UG, Hansen PB, Svenningsen P, Henriksen JE, Jensen BL. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol Dial Transpl. 2015;30:781–9.
Andersen RF, Buhl KB, Jensen BL, Svenningsen P, Friis UG, Jespersen B, et al. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. 2013;28:1227–34.
Buhl KB, Friis UG, Svenningsen P, Gulaveerasingam A, Ovesen P, Frederiksen-Møller B, et al. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension. 2012;60:1346–51.
Buhl KB, Oxlund CS, Friis UG, Svenningsen P, Bistrup C, Jacobsen IA, et al. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32:1672–7.
Chen JL, Wang L, Yao XM, Zang YJ, Wang Y, Li ZJ, et al. Association of Urinary Plasminogen-Plasmin with Edema and Epithelial Sodium Channel Activation in Patients with Nephrotic Syndrome. Am J Nephrol. 2019;50:92–104.
Hinrichs GR, Mortensen LA, Jensen BL, Bistrup C. Amiloride resolves resistant edema and hypertension in a patient with nephrotic syndrome; a case report. Physiol Rep. 2018;6:e13743.
Oxlund CS, Buhl KB, Jacobsen IA, Hansen MR, Gram J, Henriksen JE, et al. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. 2014;8:872–81.
Unruh ML, Pankratz VS, Demko JE, Ray EC, Hughey RP, Kleyman TR. Trial of Amiloride in Type 2 Diabetes with Proteinuria. Kidney Int Rep. 2017;2:893–904.
Hayata M, Kakizoe Y, Uchimura K, Morinaga J, Yamazoe R, Mizumoto T, et al. Effect of a serine protease inhibitor on the progression of chronic renal failure. Am J Physiol Ren Physiol. 2012;303:F1126–1135.
Morinaga J, Kakizoe Y, Miyoshi T, Onoue T, Ueda M, Mizumoto T, et al. The antifibrotic effect of a serine protease inhibitor in the kidney. Am J Physiol Ren Physiol. 2013;305:F173–181.
Narita Y, Ueda M, Uchimura K, Kakizoe Y, Miyasato Y, Mizumoto T, et al. Combination therapy with renin-angiotensin-aldosterone system inhibitor telmisartan and serine protease inhibitor camostat mesilate provides further renoprotection in a rat chronic kidney disease model. J Pharm Sci. 2016;130:110–6.
Ueda M, Uchimura K, Narita Y, Miyasato Y, Mizumoto T, Morinaga J, et al. The serine protease inhibitor camostat mesilate attenuates the progression of chronic kidney disease through its antioxidant effects. Nephron. 2015;129:223–32.
Edgtton KL, Gow RM, Kelly DJ, Carmeliet P, Kitching AR. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int. 2004;66:68–76.
Asami T, Tomisawa S, Uchiyama M. Effect of oral camostat mesilate on hematuria and/or proteinuria in children. Pediatr Nephrol. 2004;19:313–6.
Ikeda Y, Ito H, Hashimoto K. Effect of camostat mesilate on urinary protein excretion in three patients with advanced diabetic nephropathy. J Diabetes Complications. 1999;13:56–58.
Matsubara M, Taguma Y, Kurosawa K, Hotta O, Suzuki K, Ishizaki M. Effect of camostat mesilate for the treatment of advanced diabetic nephropathy. J Lab Clin Med. 1990;116:206–10.
Onbe T, Makino H, Kumagai I, Haramoto T, Murakami K, Ota Z. Effect of proteinase inhibitor camostat mesilate on nephrotic syndrome with diabetic nephropathy. J Diabet Complications. 1991;5:167–8.
Willis Fox O, Preston RJS. Molecular basis of protease-activated receptor 1 signaling diversity. J Thromb Haemost. 2020;18:6–16.
Ellis CA, Malik AB, Gilchrist A, Hamm H, Sandoval R, Voyno-Yasenetskaya T, et al. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J Biol Chem. 1999;274:13718–27.
Guan Y, Nakano D, Zhang Y, Li L, Liu W, Nishida M, et al. A protease-activated receptor-1 antagonist protects against podocyte injury in a mouse model of nephropathy. J Pharmacol Sci. (e-pub ahead of print 2017/11/08; https://doi.org/10.1016/j.jphs.2017.09.002) 2017.
Sato A, Nishida C, Sato-Kusubata K, Ishihara M, Tashiro Y, Gritli I, et al. Inhibition of plasmin attenuates murine acute graft-versus-host disease mortality by suppressing the matrix metalloproteinase-9-dependent inflammatory cytokine storm and effector cell trafficking. Leukemia. 2015;29:145–56.
Munakata S, Tashiro Y, Nishida C, Sato A, Komiyama H, Shimazu H, et al. Inhibition of plasmin protects against colitis in mice by suppressing matrix metalloproteinase 9-mediated cytokine release from myeloid cells. Gastroenterology. 2015;148:565–578.e564.
Acknowledgements
Q. Deng was supported by the Otsuka Toshimi Scholarship Foundation, which has enabled him to complete this project successfully. We appreciate Ms. Naoko Hirano and Kazumi Saito for their technical assistance and Ms. Noriko Nakagawa and Miki Horikiri for their secretarial assistance. The Graphical Abstract was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://smart.servier.com).
Funding
This work was supported by the Grants-in-Aid for Scientific Research KAKENHI from Japan Society for the Promotion of Science (20K17250 to T. Nakagawa and 17K09705 to Y. Kakizoe) and the Salt Science Research Foundation (20C3 to Y. Kakizoe).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, Q., Kakizoe, Y., Iwata, Y. et al. The serine protease plasmin plays detrimental roles in epithelial sodium channel activation and podocyte injury in Dahl salt-sensitive rats. Hypertens Res 46, 50–62 (2023). https://doi.org/10.1038/s41440-022-01064-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01064-2