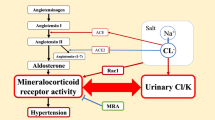
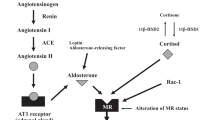
Abstract
A recent report stated that patients with primary aldosteronism who remain renin suppressed during mineralocorticoid receptor antagonist treatment might have a higher risk of developing cardiovascular disease than those with unsuppressed renin activity. We retrospectively investigated the incidence of composite cardiovascular disease and risk factors for cardiovascular disease in 1115 Japanese patients with primary aldosteronism treated with mineralocorticoid receptor antagonists. The median follow-up period was 3.0 years, and the incidence of cardiovascular events was very low (2.1%) throughout 5 years of follow-up. Changes in plasma renin activity from before to after mineralocorticoid receptor antagonist treatment were divided into three groups based on tertile, low, intermediate, and high plasma renin activity change groups, with incidences of cardiovascular disease events of 2.1%, 0.5%, and 3.7%, respectively. Multivariate Cox regression analysis revealed age (adjusted hazard ratio, 1.07; 95% confidence interval, [1.02–1.12]) and body mass index (adjusted hazard ratio, 1.13 [1.04–1.23]) as independent risk factors for cardiovascular disease. The high plasma renin activity change group had significantly higher cardiovascular disease risk with mineralocorticoid receptor antagonist treatment than the intermediate plasma renin activity change group (adjusted hazard ratio, 5.71 [1.28–25.5]). These data suggest that a high change in renin level after mineralocorticoid receptor antagonist treatment may not necessarily predict a better prognosis of cardiovascular disease in patients with primary aldosteronism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Conn JW, Cohen EL, Rovner DR. Suppression of plasma renin activity in primary aldosteronism: distinguishing primary From secondary aldosteronism in hypertensive disease. JAMA. 1964;190:213–21.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Young WF. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. Intern Med J. 2019;285:126–48.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8.
Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33.
Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–20.
Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39:1057–88.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Rossi GP, Maiolino G, Flego A, Belfiore A, Bernini G, Fabris B, et al. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71:585–91.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients With essential hypertension (ESAX-HTN study). Hypertension. 2020;75:51–8.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44:489–97.
Nakamaru R, Yamamoto K, Akasaka H, Rakugi H, Kurihara I, Yoneda T, et al. Sex differences in renal outcomes After medical treatment for bilateral primary aldosteronism. Hypertension. 2021;77:537–45.
Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism-the Japan Endocrine Society 2009. Endocr J. 2011;58:711–21.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, Van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–35.
Webb R, Mathur A, Chang R, Baid S, Nilubol N, Libutti SK, et al. What is the best criterion for the interpretation of adrenal vein sample results in patients with primary hyperaldosteronism? Ann Surg Oncol. 2012;19:1881–6.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR From serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a Selective aldosterone Blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–16.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Dhingra R, Vasan RS. Age as a cardiovascular risk factor. Med Clin North Am. 2012;96:87–91.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
James GD, Sealey JE, Müller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl. 1986;4:S387–9.
Schunkert H, Danser AH, Hense HW, Derkx FHM, Kürzinger S, Riegger GAJ. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45.
Kaplan NM, Kem DC, Holland OB, Kramer NJ, Higgins J, Gomez-Sanchez C. The intravenous furosemide test: a simple way to evaluate renin responsiveness. Ann Intern Med. 1976;84:639–45.
Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the Renin-Sodium Profile with the risk of myocardial infarction in patients with hypertension. N. Engl J Med. 1991;324:1098–104.
Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–208.
Katz FH, Roper EF. Testosterone effect on renin system in rats. Proceedings of the Society for Experimental Biology & Medicine. Proc Soc Exp Biol Med. 1977;155:330–3.
Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–63.
Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hyperten Res. 2012;35:4–16.
Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N. Engl J Med. 1981;304:930–3.
OECD. iLibrary. Health at a Glance 2019: OECD Indicators. OECD Publishing, Paris, https://doi.org/10.1787/4dd50c09-en. Accessed 19 July 2021.
Sakamoto H, Mizanur R, Nomura S, Okamoto E, Koike S, Yasunaga H, et al. (2018). World Health Organization. Regional Office for South-East Asia. Japan Health System Review. http://apps.who.int/iris/handle/10665/259941. Accessed 19 July 2021.
Acknowledgements
This study was conducted as part of the JPAS and JRAS by a Research Grant from the Japan Agency for Medical Research and Development (AMED) [grant number JP17ek0109122 and JP20ek0109352] and the National Center for Global Health and Medicine, Japan [grant number 27–1402, 30–1008]. This study was partly supported by the Research Committee on Disorders of Adrenal Hormones, a Grant-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (Nanjiseisikkanseisakukenkyujigyo [grant number 20FC1020]).
JPAS/JRAS Study Group
Hisashi Fukuda25, Yasushi Tanaka26, Yoshiyu Takeda27, Hironobu Umakoshi28, Yui Shibayama29, Takanobu Yoshimoto30, Junji Kawashima31, Katsutoshi Takahashi32, Megumi Fujita33, Minemori Watanabe34, Yuichi Matsuda35, Hirotaka Shibata36, Kohei Kamemura37, Yuichi Fujii38, Hiromi Rakugi39, Atsushi Ogo40, Shintaro Okamura41, Shozo Miyauchi42, Toshihiko Yanase43, Takashi Kawamura44, Tomikazu Fukuoka45, Tatsuya Kai46, Yuichiro Yoshikawa47, Shigeatsu Hashimoto48, Masanobu Yamada49, Ryuichi Sakamoto50, Chiba Yoshiro51
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nomura, M., Kurihara, I., Itoh, H. et al. Association of cardiovascular disease risk and changes in renin levels by mineralocorticoid receptor antagonists in patients with primary aldosteronism. Hypertens Res 45, 1476–1485 (2022). https://doi.org/10.1038/s41440-022-00960-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-00960-x
Keywords
This article is cited by
-
Prediction of endogenous mineralocorticoid receptor activity by depressor effects of mineralocorticoid receptor antagonists in patients with primary aldosteronism
Hypertension Research (2024)
-
Renin as a Biomarker to Guide Medical Treatment in Primary Aldosteronism Patients. Findings from the SPAIN-ALDO Registry
High Blood Pressure & Cardiovascular Prevention (2024)
-
Esaxerenone for nocturnal hypertension and possible future direction for treatment of hypertension-cardiovascular-kidney comorbidity
Hypertension Research (2023)
-
Importance of plasma aldosterone concentrations as a clinical indicator of nocturnal hypertension in primary aldosteronism
Hypertension Research (2023)
-
Recent progress in the diagnosis and treatment of primary aldosteronism
Hypertension Research (2023)