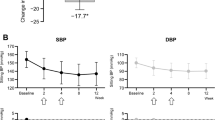
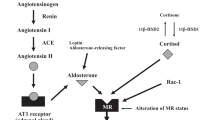
Abstract
Aldosterone (Aldo) breakthrough is a well-known phenomenon that occurs in patients with long-term renin-angiotensin aldosterone system (RAAS) blockade using inhibitors of renin or angiotensin converting enzyme or angiotensin II type 1 receptor blockers. The blockade of the mineralocorticoid receptor (MR), an Aldo binding receptor, is effective in managing patients with resistant hypertension, defined as uncontrollable blood pressure despite the concurrent use of three antihypertensive drugs. In other words, MR inhibitors are not used as first-line antihypertensive drugs in most guidelines for hypertension management. Aldo breakthrough puts hypertensive patients at higher risk of cardiovascular disease and worsens future outcomes. This review discusses Aldo secretion and the mechanism of Aldo breakthrough, dependent or independent of the RAAS, with consideration of the pharmacological aspects of this phenomenon, as well as hypothetical views.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–23.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Morimoto S, Ichihara A. Management of primary aldosteronism and mineralocorticoid receptor-associated hypertension. Hypertens Res. 2020;43:744–53.
Bravo EL. Regulation of aldosterone secretion: current concepts and newer aspects. Adv Nephrol Necker Hosp. 1977;7:105–20.
Missale C, Lombardi C, Sigala S, Spano PF. Dopaminergic regulation of aldosterone secretion. Biochemical mechanisms and pharmacology. Am J Hypertens. 1990;3:93S–5S.
Lopez AG, Duparc C, Naccache A, Castanet M, Lefebvre H, Louiset E. Role of mast cells in the control of aldosterone secretion. Horm Metab Res. 2020;52:412–20.
Boyer HG, Wils J, Renouf S, Arabo A, Duparc C, Boutelet I, et al. Dysregulation of aldosterone secretion in mast cell-deficient mice. Hypertension 2017;70:1256–63.
Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015;132:2134–45.
Xing Y, Rainey WE, Apolzan JW, Francone OL, Harris RB, Bollag WB. Adrenal cell aldosterone production is stimulated by very-low-density lipoprotein (VLDL). Endocrinology 2012;153:721–31.
Gomez-Sanchez CE, Cozza EN, Foecking MF, Chiou S, Ferris MW. Endothelin receptor subtypes and stimulation of aldosterone secretion. Hypertension 1990;15:744–7.
Rossi GP, Cavallin M, Nussdorfer GG, Pessina AC. The endothelin-aldosterone axis and cardiovascular diseases. J Cardiovasc Pharmacol. 2001;38:S49–52.
Gordon RD, Kuchel O, Liddle GW, Island DP. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Investig. 1967;46:599–605.
Wils J, Duparc C, Cailleux AF, Lopez AG, Guiheneuf C, Boutelet I, et al. The neuropeptide substance P regulates aldosterone secretion in human adrenals. Nat Commun. 2020;11:2673.
Nguyen TT, Lazure C, Babinski K, Chretien M, Ong H, De Lean A. Aldosterone secretion inhibitory factor: a novel neuropeptide in bovine chromaffin cells. Endocrinology 1989;124:1591–3.
Kawai M, Naruse M, Yoshimoto T, Naruse K, Shionoya K, Tanaka M, et al. C-type natriuretic peptide as a possible local modulator of aldosterone secretion in bovine adrenal zona glomerulosa. Endocrinology 1996;137:42–6.
Miura S, Nakayama A, Tomita S, Matsuo Y, Suematsu Y, Saku K. Comparison of aldosterone synthesis in adrenal cells, effect of various AT1 receptor blockers with or without atrial natriuretic peptide. Clin Exp Hypertens. 2015;37:353–7.
Miura SI, Suematsu Y, Matsuo Y, Tomita S, Nakayama A, Goto M, et al. The angiotensin II type 1 receptor-neprilysin inhibitor LCZ696 blocked aldosterone synthesis in a human adrenocortical cell line. Hypertens Res. 2016;39:758–63.
Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539.
Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97:434–42.
Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, et al. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76:506–16.
Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–21.
Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii T, Higaki J, et al. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:917–21.
Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids 2011;76:834–9.
Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. J Endocrinol. 1981;91:457–65.
Yoneda T, Takeda Y, Usukura M, Oda N, Takata H, Yamamoto Y, et al. Aldosterone breakthrough during angiotensin II receptor blockade in hypertensive patients with diabetes mellitus. Am J Hypertens. 2007;20:1329–33.
Sato A, Fukuda S. Effect of aldosterone breakthrough on albuminuria during treatment with a direct renin inhibitor and combined effect with a mineralocorticoid receptor antagonist. Hypertens Res. 2013;36:879–84.
Hashimoto A, Takeda Y, Karashima S, Kometani M, Aono D, Demura M, et al. Impact of mineralocorticoid receptor blockade with direct renin inhibition in angiotensin II-dependent hypertensive mice. Hypertens Res. 2020;43:1099–104.
Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pr Nephrol. 2007;3:486–92.
Schrier RW. Aldosterone ‘escape’ vs ‘breakthrough’. Nat Rev Nephrol. 2010;6:61.
Sato A, Saruta T. Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in essential hypertensive patients with left ventricular hypertrophy. J Int Med Res. 2001;29:13–21.
Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003;41:64–8.
Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 2004;47:1936–9.
Koide M, Harraz OF, Dabertrand F, Longden TA, Ferris HR, Wellman GC, et al. Differential restoration of functional hyperemia by antihypertensive drug classes in hypertension-related cerebral small vessel disease. J Clin Investig. 2021;131:e149029.
Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens. 2003;16:781–8.
Pivonello R, Ferone D, de Herder WW, de Krijger RR, Waaijers M, Mooij DM, et al. Dopamine receptor expression and function in human normal adrenal gland and adrenal tumors. J Clin Endocrinol Metab. 2004;89:4493–502.
Wu KD, Chen YM, Chu TS, Chueh SC, Wu MH, Bor-Shen H. Expression and localization of human dopamine D2 and D4 receptor mRNA in the adrenal gland, aldosterone-producing adenoma, and pheochromocytoma. J Clin Endocrinol Metab. 2001;86:4460–7.
LeHoux JG, Lefebvre A. Transcriptional activity of the hamster CYP11B2 promoter in NCI-H295 cells stimulated by angiotensin II, potassium, forskolin and bisindolylmaleimide. J Mol Endocrinol. 1998;20:183–91.
Chang HW, Chu TS, Huang HY, Chueh SC, Wu VC, Chen YM, et al. Down-regulation of D2 dopamine receptor and increased protein kinase Cmu phosphorylation in aldosterone-producing adenoma play roles in aldosterone overproduction. J Clin Endocrinol Metab. 2007;92:1863–70.
Chang HW, Wu VC, Huang CY, Huang HY, Chen YM, Chu TS, et al. D4 dopamine receptor enhances angiotensin II-stimulated aldosterone secretion through PKC-epsilon and calcium signaling. Am J Physiol Endocrinol Metab. 2008;294:E622–9.
Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert Opin Investig Drugs. 2017;26:1163–73.
Ohshima K, Mogi M, Horiuchi M. Therapeutic approach for neuronal disease by regulating renin-angiotensin system. Curr Hypertens Rev. 2013;9:99–107.
Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, et al. Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol Neurodegener. 2007;2:1.
Nakaoka H, Mogi M, Kan-No H, Tsukuda K, Ohshima K, Wang XL, et al. Angiotensin II type 2 receptor signaling affects dopamine levels in the brain and prevents binge eating disorder. J Renin Angiotensin Aldosterone Syst. 2015;16:749–57.
Naruse M, Tanabe A, Sato A, Takagi S, Tsuchiya K, Imaki T, et al. Aldosterone breakthrough during angiotensin II receptor antagonist therapy in stroke-prone spontaneously hypertensive rats. Hypertension 2002;40:28–33.
Tanabe A, Naruse M, Arai K, Naruse K, Yoshimoto T, Seki T, et al. Gene expression and roles of angiotensin II type 1 and type 2 receptors in human adrenals. Horm Metab Res. 1998;30:490–5.
Mazzocchi G, Gottardo G, Macchi V, Malendowicz LK, Nussdorfer GG. The AT2 receptor-mediated stimulation of adrenal catecholamine release may potentiate the AT1 receptor-mediated aldosterone secretagogue action of angiotensin-II in rats. Endocr Res. 1998;24:17–28.
Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, et al. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology 2011;152:1582–8.
Takeda Y, Demura M, Yoneda T, Takeda Y. DNA Methylation of the angiotensinogen gene, AGT, and the aldosterone synthase gene, CYP11B2 in cardiovascular diseases. Int J Mol Sci. 2021;22:4587.
Otani H, Otsuka F, Inagaki K, Suzuki J, Miyoshi T, Kano Y, et al. Aldosterone breakthrough caused by chronic blockage of angiotensin II type 1 receptors in human adrenocortical cells: possible involvement of bone morphogenetic protein-6 actions. Endocrinology 2008;149:2816–25.
Spat A. Glomerulosa cell–a unique sensor of extracellular K+ concentration. Mol Cell Endocrinol. 2004;217:23–6.
Kerstens MN, van der Kleij FG, Boonstra AH, Sluiter WJ, van der Molen JC, Navis G, et al. Angiotensin administration stimulates renal 11 beta-hydroxysteroid dehydrogenase activity in healthy men. Kidney Int. 2004;65:2065–70.
Adamcova M, Kawano I, Simko F. The impact of microRNAs in renin-angiotensin-system-induced cardiac remodelling. Int J Mol Sci. 2021;22:4762.
Maharjan S, Mopidevi B, Kaw MK, Puri N, Kumar A. Human aldosterone synthase gene polymorphism promotes miRNA binding and regulates gene expression. Physiol Genom. 2014;46:860–5.
Garg A, Foinquinos A, Jung M, Janssen-Peters H, Biss S, Bauersachs J, et al. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur J Heart Fail. 2020;22:1366–77.
Butterworth MB. MicroRNAs and the regulation of aldosterone signaling in the kidney. Am J Physiol Cell Physiol. 2015;308:C521–7.
Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211.
Oka T, Sakaguchi Y, Hattori K, Asahina Y, Kajimoto S, Doi Y, et al. Mineralocorticoid receptor antagonist use and hard renal outcomes in real-world patients with chronic kidney disease. Hypertension 2022;79:679–89.
Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018;17:243–60.
Ferraino KE, Cora N, Pollard CM, Sizova A, Maning J, Lymperopoulos A. Adrenal angiotensin II type 1 receptor biased signaling: The case for “biased” inverse agonism for effective aldosterone suppression. Cell Signal. 2021;82:109967.
Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci USA 2009;106:5825–30.
Sezai A, Osaka S, Yaoita H, Arimoto M, Hata H, Shiono M, et al. Changeover trial of azilsartan and olmesartan comparing effects on the renin-angiotensin-aldosterone system in patients with essential hypertension after cardiac surgery (CHAOS Study). Ann Thorac Cardiovasc Surg. 2016;22:161–7.
Sezai A, Soma M, Hata M, Yoshitake I, Unosawa S, Wakui S, et al. Effects of olmesartan on the renin-angiotensin-aldosterone system for patients with essential hypertension after cardiac surgery–investigation using a candesartan change-over study. Ann Thorac Cardiovasc Surg. 2011;17:487–93.
Tsutamoto T, Nishiyama K, Yamaji M, Kawahara C, Fujii M, Yamamoto T, et al. Comparison of the long-term effects of candesartan and olmesartan on plasma angiotensin II and left ventricular mass index in patients with hypertension. Hypertens Res. 2010;33:118–22.
Eklind-Cervenka M, Benson L, Dahlstrom U, Edner M, Rosenqvist M, Lund LH. Association of candesartan vs losartan with all-cause mortality in patients with heart failure. JAMA 2011;305:175–82.
Svanstrom H, Pasternak B, Hviid A. Association of treatment with losartan vs candesartan and mortality among patients with heart failure. JAMA 2012;307:1506–12.
Toth AD, Prokop S, Gyombolai P, Varnai P, Balla A, Gurevich VV, et al. Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by beta-arrestins. J Biol Chem. 2018;293:876–92.
Sun N, Kim KM. Mechanistic diversity involved in the desensitization of G protein-coupled receptors. Arch Pharm Res. 2021;44:342–53.
Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, et al. Effect of repetitive icv injections of ANG II on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1095–104.
Vento PJ, Daniels D. Mitogen-activated protein kinase is required for the behavioural desensitization that occurs after repeated injections of angiotensin II. Exp Physiol. 2012;97:1305–14.
Calebiro D, Nikolaev VO, Lohse MJ. Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J Mol Endocrinol. 2010;45:1–8.
Burton JC, Grimsey NJ. Ubiquitination as a key regulator of endosomal signaling by GPCRs. Front Cell Dev Biol. 2019;7:43.
Fujita K, Aguilera G, Catt KJ. The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cells. J Biol Chem. 1979;254:8567–74.
Giubilaro J, Schuetz DA, Stepniewski TM, Namkung Y, Khoury E, Lara-Marquez M, et al. Discovery of a dual Ras and ARF6 inhibitor from a GPCR endocytosis screen. Nat Commun. 2021;12:4688.
Bhuiyan MA, Hossain M, Nakamura T, Ozaki M, Nagatomo T. Internalization of constitutively active N111G MUTANT of AT1 receptor induced by angiotensin II-receptor antagonists candesartan, losartan, and telmisartan: comparison with valsartan. J Pharm Sci. 2010;112:459–62.
Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014;64:1368–75.
Rukavina Mikusic NL, Silva MG, Pineda AM, Gironacci MM. Angiotensin receptors heterodimerization and trafficking: how much do they influence their biological function? Front Pharmacol. 2020;11:1179.
Martinez-Pinilla E, Rodriguez-Perez AI, Navarro G, Aguinaga D, Moreno E, Lanciego JL, et al. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem Pharmacol. 2015;96:131–42.
Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Investig. 2008;118:2180–9.
O’Connor PJ, Sperl-Hillen JAM, Johnson PE, Rush WA, Biltz G. Clinical inertia and outpatient medical errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). https://www.ncbi.nlm.nih.gov/pubmed/21249838.): Rockville (MD), 2005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mogi, M. Aldosterone breakthrough from a pharmacological perspective. Hypertens Res 45, 967–975 (2022). https://doi.org/10.1038/s41440-022-00913-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-00913-4
Keywords
This article is cited by
-
Clues to suspect aldosterone breakthrough in the real world
Hypertension Research (2023)
-
Aldosterone breakthrough from a pharmacological perspective
Hypertension Research (2023)
-
Reply to comments about “Aldosterone breakthrough”
Hypertension Research (2023)
-
Inhibition of aldosterone synthase: Does this offer advantages compared with the blockade of mineralocorticoid receptors?
Hypertension Research (2023)
-
Aldosterone breakthrough as a clue to the physiological importance of paracrine regulation of aldosterone secretion
Hypertension Research (2022)