Abstract
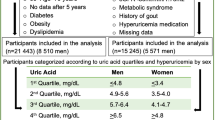
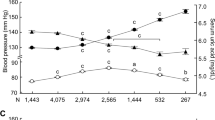
Whether hyperuricemia is a true risk factor for elevated blood pressure (BP) is controversial, and the sex-specific effects of serum uric acid (SUA) on BP during a follow-up period remain unclear. We investigated whether the association of SUA level with systolic or diastolic BP during a 10-year period differs by sex in a Japanese general population of individuals who received annual health examinations (n = 28,990). After exclusion of subjects who had no BP or SUA data at baseline, a total of 22,994 subjects (male/female: 14,603/8391, age: 47 ± 11 years) were recruited. After adjustment for age; body mass index; BP; SUA level; use of drugs for hyperuricemia and hypertension; diagnosis of diabetes mellitus, dyslipidemia, and chronic kidney disease; family history of hypertension; habits of current smoking and alcohol consumption at baseline; the duration of the observation period; and the interaction between each covariate and the duration of the observation period indicated a significant association of SUA level with change in systolic or diastolic BP over time. There was a significant interaction between sex and SUA level for the change in systolic BP (P = 0.003) but not the change in diastolic BP (P = 0.081). The SUA level at baseline (per 1 mg/dL) was significantly associated with a change in systolic BP over time in females (estimate: 0.073 mmHg/year, P = 0.003) but not in males (estimate: 0.020 mmHg/year, P = 0.160). In conclusion, a high SUA level at baseline is significantly associated with an increase in systolic BP over time in female individuals but not in male individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Furuhashi M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am J Physiol Endocrinol Metab. 2020;319:E827–E34.
Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017;357:j2376.
Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 2007;49:298–303.
Nishio S, Maruyama Y, Sugano N, Hosoya T, Yokoo T, Kuriyama S. Gender interaction of uric acid in the development of hypertension. Clin Exp Hypertens. 2018;40:446–51.
Gaffo AL, Jacobs DR Jr, Sijtsma F, Lewis CE, Mikuls TR, Saag KG. Serum urate association with hypertension in young adults: analysis from the Coronary Artery Risk Development in Young Adults cohort. Ann Rheum Dis. 2013;72:1321–7.
Kuwabara M, Niwa K, Hisatome I, Nakagawa T, Roncal-Jimenez CA, Andres-Hernando A, et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five-year japanese cohort study. Hypertension 2017;69:1036–44.
Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, et al. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One. 2014;9:e114259.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63:102–10.
Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–42.
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8:e56864.
Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122.
Mori K, Furuhashi M, Tanaka M, Numata K, Hisasue T, Hanawa N, et al. U-shaped relationship between serum uric acid level and decline in renal function during a 10-year period in female subjects: BOREAS-CKD2. Hypertens Res. 2021;44:107–16.
Akasaka H, Yoshida H, Takizawa H, Hanawa N, Tobisawa T, Tanaka M, et al. The impact of elevation of serum uric acid level on the natural history of glomerular filtration rate (GFR) and its sex difference. Nephrol Dial Transpl. 2014;29:1932–9.
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62:170–80.
De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis 2010;69:1162–4.
Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16:15–24.
Barbieri L, Verdoia M, Schaffer A, Marino P, Suryapranata H, De Luca G, et al. Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: A single-centre study. Atherosclerosis 2015;241:241–8.
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001;38:1101–6.
Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–47.
Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–40.
Joosten LAB, Crisan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol. 2020;16:75–86.
Jordan DM, Choi HK, Verbanck M, Topless R, Won HH, Nadkarni G, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study. PLoS Med. 2019;16:e1002725.
Pfister R, Barnes D, Luben R, Forouhi NG, Bochud M, Khaw KT, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia 2011;54:2561–9.
Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–30.
Johnson RJ, Merriman T, Lanaspa MA. Causal or noncausal relationship of uric acid with diabetes. Diabetes 2015;64:2720–2.
Nishino T, Okamoto K. Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. J Biol Inorg Chem. 2015;20:195–207.
Chaves FJ, Corella D, Blesa S, Mansego ML, Marin P, Portoles O, et al. Xanthine oxidoreductase polymorphisms: influence in blood pressure and oxidative stress levels. Pharmacogenet Genomics. 2007;17:589–96.
Yang J, Kamide K, Kokubo Y, Takiuchi S, Horio T, Matayoshi T, et al. Associations of hypertension and its complications with variations in the xanthine dehydrogenase gene. Hypertens Res. 2008;31:931–40.
Wu B, Hao Y, Shi J, Geng N, Li T, Chen Y, et al. Association between xanthine dehydrogenase tag single nucleotide polymorphisms and essential hypertension. Mol Med Rep. 2015;12:5685–90.
Scheepers LE, Wei FF, Stolarz-Skrzypek K, Malyutina S, Tikhonoff V, Thijs L, et al. Xanthine oxidase gene variants and their association with blood pressure and incident hypertension: a population study. J Hypertens. 2016;34:2147–54.
Stewart DJ, Langlois V, Noone D. Hyperuricemia and hypertension: links and risks. Integr Blood Press Control. 2019;12:43–62.
Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National health and nutrition examination survey. JAMA 2000;283:2404–10.
Mikkelsen WM, Dodge HJ, Valkenburg H. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959-1960. Am J Med. 1965;39:242–51.
Furuhashi M, Mori K, Tanaka M, Maeda T, Matsumoto M, Murase T, et al. Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr J. 2018;65:1083–92.
Hosoyamada M, Takiue Y, Shibasaki T, Saito H. The effect of testosterone upon the urate reabsorptive transport system in mouse kidney. Nucleosides Nucleotides Nucleic Acids. 2010;29:574–9.
Takiue Y, Hosoyamada M, Kimura M, Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids. 2011;30:113–9.
Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martinez G, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93:2230–3.
Yoshitomi R, Fukui A, Nakayama M, Ura Y, Ikeda H, Oniki H, et al. Sex differences in the association between serum uric acid levels and cardiac hypertrophy in patients with chronic kidney disease. Hypertens Res. 2014;37:246–52.
Nagasawa Y, Yamamoto R, Shoji T, Shinzawa M, Hasuike Y, Nagatoya K, et al. Serum Uric Acid Level Predicts Progression of IgA Nephropathy in Females but Not in Males. PLoS One. 2016;11:e0160828.
Matsukuma Y, Masutani K, Tanaka S, Tsuchimoto A, Fujisaki K, Torisu K, et al. A J-shaped association between serum uric acid levels and poor renal survival in female patients with IgA nephropathy. Hypertens Res. 2017;40:291–7.
Oh TR, Choi HS, Kim CS, Kang KP, Kwon YJ, Kim SG, et al. The effects of hyperuricemia on the prognosis of IgA nephropathy are more potent in females. J Clin Med. 2020;9:176.
Oh TR, Choi HS, Kim CS, Ryu DR, Park SH, Ahn SY, et al. Serum uric acid is associated with renal prognosis of lupus nephritis in women but not in men. J Clin Med. 2020;9:773.
Takahashi S, Tanaka M, Furuhashi M, Moniwa N, Koyama M, Higashiura Y, et al. Fatty liver index is independently associated with deterioration of renal function during a 10-year period in healthy subjects. Sci Rep. 2021;11:8606.
Higashiura Y, Tanaka M, Furuhashi M, Koyama M, Ohnishi H, Numata K, et al. Low urine pH predicts new onset of diabetes mellitus during a 10-year period in men: BOREAS-DM1 study. J Diabetes Investig. 2020;11:1490–7.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11–S24.
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47.
Leffondre K, Boucquemont J, Tripepi G, Stel VS, Heinze G, Dunkler D. Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol Dial Transpl. 2015;30:1237–43.
Janmaat CJ, van Diepen M, Tsonaka R, Jager KJ, Zoccali C, Dekker FW. Pitfalls of linear regression for estimating slopes over time and how to avoid them by using linear mixed-effects models. Nephrol Dial Transpl. 2019;34:561–6.
Shieh G. Clarifying the role of mean centring in multicollinearity of interaction effects. Br J Math Stat Psychol. 2011;64:462–77.
Franklin SS, Gustin WT, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, et al. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res. 2013;36:43–9.
Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42.
Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–6.
Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean Platelet Volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074.
Coban E, Yazicioglu G, Berkant Avci A, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets 2005;16:435–8.
Kaya MG, Yarlioglues M, Gunebakmaz O, Gunturk E, Inanc T, Dogan A, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis 2010;209:278–82.
Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Gender differences in the relationship between serum uric acid and mean platelet volume in a Japanese general population. Platelets 2014;25:202–6.
Furuhashi M, Matsumoto M, Tanaka M, Moniwa N, Murase T, Nakamura T, et al. Plasma Xanthine Oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ J. 2018;82:1892–9.
Furuhashi M, Matsumoto M, Murase T, Nakamura T, Higashiura Y, Koyama M, et al. Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J Diabetes Investig. 2019;10:1059–67.
Furuhashi M, Koyama M, Matsumoto M, Murase T, Nakamura T, Higashiura Y, et al. Annual change in plasma xanthine oxidoreductase activity is associated with changes in liver enzymes and body weight. Endocr J. 2019;66:777–86.
Furuhashi M, Koyama M, Higashiura Y, Murase T, Nakamura T, Matsumoto M, et al. Differential regulation of hypoxanthine and xanthine by obesity in a general population. J Diabetes Investig. 2020;11:878–87.
Yoshida S, Kurajoh M, Fukumoto S, Murase T, Nakamura T, Yoshida H, et al. Association of plasma xanthine oxidoreductase activity with blood pressure affected by oxidative stress level: MedCity21 health examination registry. Sci Rep. 2020;10:4437.
Furuhashi M, Higashiura Y, Koyama M, Tanaka M, Murase T, Nakamura T, et al. Independent association of plasma xanthine oxidoreductase activity with hypertension in nondiabetic subjects not using medication. Hypertens Res. 2021;44:1213–20.
Pineda C, Soto-Fajardo C, Mendoza J, Gutierrez J, Sandoval H. Hypouricemia: what the practicing rheumatologist should know about this condition. Clin Rheumatol. 2020;39:135–47.
Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391–404.
Acknowledgements
The authors are grateful to Keita Numata and Takashi Hisasue for data management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mori, K., Furuhashi, M., Tanaka, M. et al. Serum uric acid level is associated with an increase in systolic blood pressure over time in female subjects: Linear mixed-effects model analyses. Hypertens Res 45, 344–353 (2022). https://doi.org/10.1038/s41440-021-00792-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00792-1
Keywords
This article is cited by
-
An analysis of the associations of high-sensitivity C-reactive protein and uric acid with metabolic syndrome components in Korean adults by sex: a cross-sectional study using the Korea national health and nutrition examination survey 2016–2018
BMC Endocrine Disorders (2023)
-
Preface—this month’s Asian perspectives
Hypertension Research (2023)
-
Metabolic dysfunction-associated fatty liver disease is associated with an increase in systolic blood pressure over time: linear mixed-effects model analyses
Hypertension Research (2023)
-
An increase in calculated small dense low-density lipoprotein cholesterol predicts new onset of hypertension in a Japanese cohort
Hypertension Research (2023)
-
Sex-specific associations between serum uric acid levels and risk of hypertension for different diagnostic reference values of high blood pressure
Hypertension Research (2023)