Abstract
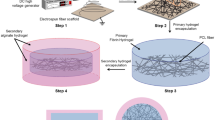
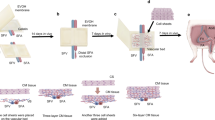
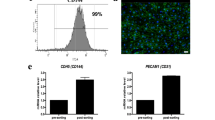
Rapid blood vessel ingrowth into transplanted constructs represents the key requirement for successful tissue engineering. Seeding three-dimensional scaffolds with suitable cells is an approved technique for this challenge. Since a plethora of patients suffer from widespread diseases that limit the capacity of neoangiogenesis (e.g., hypertension), we investigated the incorporation of cell-seeded poly-L-lactide-co-glycolide scaffolds in hypertensive (BPH/2J, group A) and nonhypertensive (BPN/3J, group B) mice. Collagen-coated scaffolds (A1 and B1) were additionally seeded with osteoblast-like (A2 and B2) and mesenchymal stem cells (A3 and B3). After implantation into dorsal skinfold chambers, inflammation and newly formed microvessels were measured using repetitive intravital fluorescence microscopy for 2 weeks. Apart from a weak inflammatory response in all groups, significantly increased microvascular densities were found in cell-seeded scaffolds (day 14, A2: 192 ± 12 cm/cm2, A3: 194 ± 10 cm/cm2, B2: 249 ± 19 cm/cm2, B3: 264 ± 17 cm/cm2) when compared with controls (A1: 129 ± 10 cm/cm2, B1: 185 ± 8 cm/cm2). In this context, hypertensive mice showed reduced neoangiogenesis in comparison with nonhypertensive animals. Therefore, seeding approved scaffolds with organ-specific or pluripotent cells is a very promising technique for tissue engineering in hypertensive organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Frerich B, Lindemann N, Kurtz-Hoffmann J, Oertel K. In vitro model of a vascular stroma for the engineering of vascularized tissues. Int J Clin Oral Maxillofac Surg. 2001;30:414–20.
Kampmann A, Lindhorst D, Schumann P, Zimmerer R, Kokemuller H, Rucker M, et al. Additive effect of mesenchymal stem cells and VEGF to vascularization of PLGA scaffolds. Microvasc Res. 2013;90:71–79.
Lindhorst D, Tavassol F, von See C, Schumann P, Laschke MW, Harder Y, et al. Effects of VEGF loading on scaffold-confined vascularization. J Biomed Mater Res A. 2010;95:783–92.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, et al. Endothelialized networks with a vascular geometry in microfabricated poly(dimethyl siloxane). Biomed Microdevices. 2004;6:269–78.
Rubenstein D, Han D, Goldgraben S, El-Gendi H, Gouma PI, Frame MD. Bioassay chamber for angiogenesis with perfused explanted arteries and electrospun scaffolding. Microcirculation. 2007;14:723–37.
Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–9.
Levenberg S, Langer R. Advances in tissue engineering. Curr Top Dev Biol. 2004;61:113–34.
Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–20.
Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19.
Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–9.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60.
Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8.
Neuhauser H, Thamm M, Ellert U. Blutdruck in Deutschland 2008–11. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:795–801.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544.
Levy BI, Schiffrin EL, Mourad J-J, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–76.
Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–7.
Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibiriçà E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens. 2006;19:477–83.
Kubis N, Richer C, Domergue V, Giudicelli J-F, Lévy BI. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt−/−). J Hypertens. 2002;20:1581–7.
de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–35.
Fukuda S, Yasu T, Kobayashi N, Ikeda N, Schmid-Schönbein GW. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95:100–8.
Schumann P, Tavassol F, Lindhorst D, Stuehmer C, Bormann KH, Kampmann A, et al. Consequences of seeded cell type on vascularization of tissue engineering constructs in vivo. Microvasc Res. 2009;78:180–90.
Schumann P, von See C, Kampmann A, Lindhorst D, Tavassol F, Kokemuller H, et al. Comparably accelerated vascularization by preincorporation of aortic fragments and mesenchymal stem cells in implanted tissue engineering constructs. J Biomed Mater Res A. 2011;97:383–94.
Schumann P, Lindhorst D, von See C, Menzel N, Kampmann A, Tavassol F, et al. Accelerating the early angiogenesis of tissue engineering constructs in vivo by the use of stem cells cultured in matrigel. J Biomed Mater Res A. 2014;102:1652–62.
Tavassol F, Kampmann A, Lindhorst D, Schumann P, Kokemuller H, Bormann KH, et al. Prolongated survival of osteoblast-like cells on biodegradable scaffolds by heat shock preconditioning. Tissue Eng Part A. 2011;17:1935–43.
Tavassol F, Schumann P, Lindhorst D, Sinikovic B, Voss A, von See C, et al. Accelerated angiogenic host tissue response to poly(L-lactide-co-glycolide) scaffolds by vitalization with osteoblast-like cells. Tissue Eng Part A. 2010;16:2265–79.
Schumann P, Kampmann A, Sauer G, Lindhorst D, von See C, Stoetzer M, et al. Accelerated vascularization of tissue engineering constructs in vivo by preincubated co-culture of aortic fragments and osteoblasts. Biochem Eng J. 2016;105:230–41.
Schumann P, Lindhorst D, Kampmann A, Gellrich NC, Krone-Wolf S, Meyer-Lindenberg A, et al. Decelerated vascularization in tissue-engineered constructs in association with diabetes mellitus in vivo. J Diabetes Complicat. 2015;29:855–64.
Schlager G, Sides J. Characterization of hypertensive and hypotensive inbred strains of mice. Lab Anim Sci. 1997;47:288–92.
Uddin M, Yang H, Shi M, Polley-Mandal M, Guo Z. Elevation of oxidative stress in the aorta of genetically hypertensive mice. Mech Ageing Dev. 2003;124:811–7.
Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–62.
Carvalho C, Landers R, Hübner U, Schmelzeisen R, Mülhaupt R. Fabrication of soft and hard biocompatible scaffolds using 3D-BioplottingTM. Virtual modeling and rapid manufacturing-advanced research in virtual and rapid prototyping. London: Taylor & Francis Group; 2005, pp 97–102.
Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, et al. Expression patterns of bone‐related proteins during osteoblastic differentiation in MC3T3‐E1 cells. J Cell Biochem. 1996;61:609–18.
Park B-W, Hah Y-S, Choi M-J, Ryu Y-M, Lee S-G, Kim DR, et al. In vitro osteogenic differentiation of cultured human dental papilla-derived cells. J Oral Maxillofac Surg. 2009;67:507–14.
Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606–7.
Meirelles LdS, Nardi NB. Murine marrow‐derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–11.
Rucker M, Schafer T, Roesken F, Spitzer WJ, Bauer M, Menger MD. Reduction of inflammatory response in composite flap transfer by local stress conditioning-induced heat-shock protein 32. Surgery. 2001;129:292–301.
Laschke MW, Witt K, Pohlemann T, Menger MD. Injectable nanocrystalline hydroxyapatite paste for bone substitution: in vivo analysis of biocompatibility and vascularization. J Biomed Mater Res B Appl Biomater. 2007;82:494–505.
Baker M, Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res. 1974;7:131–43.
Menger MD, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: significance of “no reflow”. Am J Physiol. 1992;263:H1892–900.
Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–79.
Selkurt EE, Abel FL, Edwards JL, Yum M. Renal function in dogs with hypertension induced by immunologic nephritis. Proc Soc Exp Biol Med. 1973;144:295–303.
Spitznagel JK, Schroeder HA. Experimental pyelonephritis and hypertension in rats. Proc Soc Exp Biol Med. 1951;77:762–4.
Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension. 2002;39:1101–6.
Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146:160–73.
Marques FZ, Campain AE, Davern PJ, Yang YHJ, Head GA, Morris BJ. Global identification of the genes and pathways differentially expressed in hypothalamus in early and established neurogenic hypertension. Physiol Genomics. 2011;43:766–71.
Jackson KL, Marques FZ, Watson AM, Palma-Rigo K, Nguyen-Huu T-P, Morris BJ, et al. A novel interaction between sympathetic overactivity and aberrant regulation of renin by miR-181a in BPH/2J genetically hypertensive mice. Hypertension. 2013;62:775–81.
Sugiyama F, Yagami K, Paigen B. Mouse models of blood pressure regulation and hypertension. Curr Hypertens Rep. 2001;3:41–8.
Chiu CL, Jackson KL, Hearn NL, Steiner N, Head GA, Lind JM. Identification of genes with altered expression in male and female Schlager hypertensive mice. BMC Med Genet. 2014;15:101.
Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–104.
Rücker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mülhaupt R, et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27:5027–38.
Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65–80.
Au P, Tam J, Duda DG, Lin P-C, Munn LL, Fukumura D, et al. Paradoxical effects of PDGF-BB overexpression in endothelial cells on engineered blood vessels in vivo. Am J Pathol. 2009;175:294–302.
Erman H, Gelisgen R, Cengiz M, Tabak O, Erdenen F, Uzun H. The association of vascular endothelial growth factor, metalloproteinases and their tissue inhibitors with cardiovascular risk factors in the metabolic syndrome. Eur Rev Med Pharm Sci. 2016;20:1015–22.
Ferroni P, Della-Morte D, Palmirotta R, Rundek T, Guadagni F, Roselli M. Angiogenesis and hypertension: the dual role of anti-hypertensive and anti-angiogenic therapies. Curr Vasc Pharm. 2012;10:479–93.
Kaess BM, Preis SR, Beiser A, Sawyer DB, Chen TC, Seshadri S, et al. Circulating vascular endothelial growth factor and the risk of cardiovascular events. Heart. 2016;102:1898–901.
Rüger BM, Breuss J, Hollemann D, Yanagida G, Fischer MB, Mosberger I, et al. Vascular morphogenesis by adult bone marrow progenitor cells in three‐dimensional fibrin matrices. Differentiation. 2008;76:772–83.
Deckers MM, Van Bezooijen RL, Van Der Horst G, Hoogendam J, van der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–53.
Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–26.
Schlager GJG. Selection for blood pressure levels in mice. Genetics. 1974;76:537–49.
Zhang B, Xie Q-Y, Quan Y, Pan X-M, Liam D-F. Reactive oxygen species induce cell death via Akt signaling in rat osteoblast-like cell line ROS 17/2.8. Toxicol Ind Health. 2015;31:1236–42.
Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–14.
Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med. 2001;226:507–20.
Chopinaud M, Labbé D, Creveuil C, Marc M, Bénateau H, Mourgeon B, et al. Autologous adipose tissue graft to treat hypertensive leg ulcer: a pilot study. Dermatology. 2017;233:234–41.
Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960.
Laschke M, Häufel J, Thorlacius H, Menger M. New experimental approach to study host tissue response to surgical mesh materials in vivo. J Biomed Mater Res A. 2005;74:696–704.
Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33:89–94.
Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–41.
Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–50.
Bonnin P, Vilar J, Levy BI. Effect of normovolemic hematocrit changes on blood pressure and flow. Life Sci. 2016;157:62–66.
Jackson KL, Head GA, Gueguen C, Stevenson ER, Lim JK, Marques FJFip. Mechanisms responsible for genetic hypertension in Schlager BPH/2 mice. Front Physiol. 2019;10:1311.
Baumbach GL, Sigmund CD, Faraci FMJH. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55.
McGuire JJ, Van Vliet BN, Giménez J, King JC, Halfyard SJ. Persistence of PAR-2 vasodilation despite endothelial dysfunction in BPH/2 hypertensive mice. Pflug Arch. 2007;454:535–43.
Nelson JW, Ferdaus MZ, McCormick JA, Minnier J, Kaul S, Ellison DH, et al. Endothelial transcriptomics reveals activation of fibrosis-related pathways in hypertension. Physiol Genomics. 2018;50:104–16.
Tajada S, Cidad P, Moreno‐Domínguez A, Pérez‐García MT, López‐López JR. High blood pressure associates with the remodelling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J Physiol. 2012;590:6075–91.
Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–7.
Fan J-S, Zhang Q, Li M, Tochio H, Yamazaki T, Shimizu M, et al. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J Biol Chem. 1998;273:33472–81.
Antonios T, Rattray F, Singer D, Markandu N, Mortimer P, MacGregor G. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–8.
Pries AR, Secomb TW, Gaehtgens P. Structural autoregulation of terminal vascular beds: vascular adaptation and development of hypertension. Hypertension. 1999;33:153–61.
Levy B, Ambrosio G, Pries A, Struijker-Boudier H. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–40.
Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, et al. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–8.
Houben A, Canoy M, Paling HA, Derhaag PJ, de Leeuw PW. Quantitative analysis of retinal vascular changes in essential and renovascular hypertension. J Hypertens. 1995;13:1729–33.
Sala A, Hänseler P, Ranga A, Lutolf MP, Vörös J, Ehrbar M, et al. Engineering 3D cell instructive microenvironments by rational assembly of artificial extracellular matrices and cell patterning. Integr Biol. 2011;3:1102–11.
Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, et al. Endothelialized networks with a vascular geometry in microfabricated poly (dimethyl siloxane). Biomed Microdevices. 2004;6:269–78.
Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharm. 2007;7:151–7.
Kobayashi N, DeLano FA, Schmid-Schönbein GW. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2005;25:2114–21.
Saheera S, Nair RR. Accelerated decline in cardiac stem cell efficiency in Spontaneously hypertensive rat compared to normotensive Wistar rat. PLoS ONE. 2017;12:e0189129
Saheera S, Potnuri AG, Nair RR. Modulation of cardiac stem cell characteristics by metoprolol in hypertensive heart disease. Hypertens Res. 2018;41:253–62.
Saheera S, Potnuri AG, Nair RR. Protective effect of antioxidant Tempol on cardiac stem cells in chronic pressure overload hypertrophy. Life Sci. 2019;222:88–93.
Acknowledgements
The authors are grateful for the excellent technical assistance of Marie Luise Jenzer and Stefanie Rausch.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wagner, M.E.H., Kampmann, A., Schumann-Moor, K. et al. Cell seeding accelerates the vascularization of tissue engineering constructs in hypertensive mice. Hypertens Res 44, 23–35 (2021). https://doi.org/10.1038/s41440-020-0524-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0524-z