Abstract
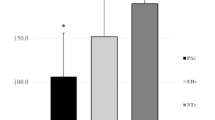
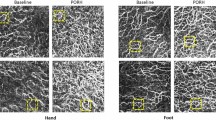
The aim of our cross-sectional study was to evaluate skin microvascular alterations in patients with hypertension secondary to primary aldosteronism (PA) and in subjects with essential hypertension (EH). Skin microcirculation was detected by nailfold videocapillaroscopy (NVC) and laser Doppler perfusion imaging (LDPI), both noninvasive techniques for the evaluation of digital capillaroscopic damage and hand skin blood perfusion. From September 2018 to April 2019, we consecutively enrolled 80 patients, of whom 42 had PA and 38 had EH. A morphological and structural study of cutaneous microcirculation was carried out through NVC, while functional evaluation of the peripheral microcirculation was carried out with LDPI. Using LDPI indices, dermal perfusion gradients were calculated in various regions of interest at the level of the back of the hand (ROI1 and ROI2). No differences between the two groups in NVC parameters were found. In contrast, LDPI showed worse skin perfusion parameters in patients with PA compared with patients with EH (ROI1: 143.9 ± 29.9 pU vs 163.3 ± 35.2 pU, p = 0.01; perfusion gradient ROI1–ROI2: 62.4 ± 28.8 pU vs 79.3 ± 33.5 pU, p = 0.019). Furthermore, the ROI1–ROI2 (proximal-distal) perfusion gradient was negatively correlated with aldosterone plasma levels (r −0.269; p = 0.017). Multivariate analysis showed that aldosterone was significantly associated with the ROI1–ROI2 perfusion gradient (b −0.220; p = 0.044). Patients with PA showed altered skin perfusion and greater microvascular dysfunction compared with the EH group. Our results are consistent with the hypothesis that aldosterone may have a pathophysiological role in microvascular remodeling in patients with PA, with predominant functional dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C.PAPY Study Investigators et al. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248.
Strauch B, Petrák O, Zelinka T, Wichterle D, Holaj R, Kasalický M, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21:1086–92.
Rizzoni D, Paiardi S, Rodella L, Porteri E, De Ciuceis C, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J Clin Endocrinol Metab. 2006;91:2638–42.
Holaj R, Rosa J, Zelinka T, Štrauch B, Petrák O, Indra T, et al. Long-term effect of specific treatment of primary aldosteronism on carotid intima-media thickness. J Hypertens. 2015;33:874–82.
Prejbisz A, Warchoł-Celińska E, Lenders JW, Januszewicz A. Cardiovascular risk in primary hyperaldosteronism. Horm Metab Res. 2015;47:973–80.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–06.
Rosa J, Somlóová Z, Petrák O, Strauch B, Indra T, Senitko M, et al. Peripheral arterial stiffness in primary aldosteronism. Physiol Res. 2012;61:461–8.
Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–5.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. ESC Scientific Document Group
Ceruti M, Petramala L, Cotesta D, Cerci S, Serra V, Caliumi C, et al. Ambulatory blood pressure monitoring in secondary arterial hypertension due to adrenal diseases. J Clin Hypertens. 2006;8:642–8.
Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27:155–60.
CRABBE J. Stimulation of active sodium transport by the isolated toad bladder with aldosterone in vitro. J Clin Investig. 1961;40:2103–10.
Ebata S, Muto S, Okada K, Nemoto J, Amemiya M, Saito T, et al. Aldosterone activates Na+/H+ exchange in vascular smooth muscle cells by nongenomic and genomic mechanisms. Kidney Int. 1999;56:1400–12.
Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9.
Petramala L, Pignatelli P, Carnevale R, Zinnamosca L, Marinelli C, Settevendemmie A, et al. Oxidative stress in patients affected by primary aldosteronism. J Hypertens. 2014;32:2022–9.
Gkaliagkousi E, Anyfanti P, Triantafyllou A, Gavriilaki E, Nikolaidou B, Lazaridis A, et al. Aldosterone as a mediator of microvascular and macrovascular damage in a population of normotensive to early-stage hypertensive individuals. J Am Soc Hypertens. 2018;12:50–7.
Fritsch Neves M, Schiffrin EL. Aldosterone: a risk factor for vascular disease. Curr Hypertens Rep. 2003;5:59–65.
Varano M, Iacono P, Tedeschi MM, Letizia C, Curione M, Savoriti C, et al. Comparisons of microvascular and macrovascular changes in aldosteronism-related hypertension and essential hypertension. Sci Rep. 2017;7:2666.
Wu VC, Yang SY, Lin JW, Cheng BW, Kuo CC, Tsai CT, et al. Kidney impairment in primary aldosteronism. Clin Chim Acta. 2011;412:1319–25. TAIPAI Study Group
Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16:1320–5.
Kishimoto S, Matsumoto T, Oki K, Maruhashi T, Kajikawa M, Matsui S, et al. Microvascular endothelial function is impaired in patients with idiopathic hyperaldosteronism. Hypertens Res. 2018;41:932–8.
Gafane-Matemane LF, van Rooyen JM, Schutte R, Schutte AE. Aldosterone and renin in relation to surrogate measures of sympathetic activity: the SABPA study. Cardiovasc J Afr. 2019;30:34–40.
Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99.
Queisser N, Schupp N. Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic Biol Med. 2012;53:314–27.
Chun TY, Bloem LJ, Pratt JH. Aldosterone inhibits inducible nitric oxide synthase in neonatal rat cardiomyocytes. Endocrinology. 2003;144:1712–7.
Young MJ. Mechanisms of mineralocorticoid receptor-mediated cardiac fibrosis and vascular inflammation. Curr Opin Nephrol Hypertens. 2008;17:174–80.
Juknevicius I, Segal Y, Kren S, Lee R, Hostetter TH. Effect of aldosterone on renal transforming growth factor-beta. Am J Physiol Ren Physiol. 2004;286:F1059–1062.
Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am J Hypertens. 2004;17:597–603.
Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–71.
Junqueira CLC, Magalhães MEC, Brandão AA, Ferreira E, Cyrino FZGA, Maranhão PA. et al. Microcirculation and biomarkers in patients with resistant or mild-to-moderate hypertension: a cross-sectional study. Hypertens Res. 2018;41:515–23.
Ruaro B, Smith V, Sulli A, Pizzorni C, Tardito S, Patané M, et al. Innovations in the assessment of primary and secondary Raynaud’s phenomenon. Front Pharmacol. 2019;16:10:360.
Ruaro B, Sulli A, Pizzorni C, Paolino S, Smith V, Cutolo M. Correlations between skin blood perfusion values and nailfold capillaroscopy scores in systemic sclerosis patients. Microvasc Res. 2016;105:119–24.
Herrick AL, Murray A. The role of capillaroscopy and thermography in the assessment and management of Raynaud’s phenomenon. Autoimmun Rev. 2018;17:465–72.
Wigley FM, Wise RA, Mikdashi J, Schaefer S, Spence RJ. The post-occlusive hyperemic response in patients with systemic sclerosis. Arthr Rheum. 1990;33:1620–5.
Clark S, Dunn G, Moore T, Jayson M, King TA, Herrick AL. Comparison of thermography and laser Doppler imaging in the assessment of Raynaud’s phenomenon. Microvasc Res. 2003;66:73–6.
Murray AK, Moore TL, Manning JB, Taylor C, Griffiths CE, Herrick AL. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthr Rheum. 2009;61:1103–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper. All authors have approved the manuscript in the present form. This study was not funded. The manuscript is not under consideration for publication elsewhere. All authors give final approval of the version to be submitted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Concistrè, A., Petramala, L., Bonvicini, M. et al. Comparisons of skin microvascular changes in patients with primary aldosteronism and essential hypertension. Hypertens Res 43, 1222–1230 (2020). https://doi.org/10.1038/s41440-020-0475-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0475-4
Keywords
This article is cited by
-
Assessment of skin microcirculation in primary aldosteronism: impaired microvascular responses compared to essential hypertensives and normotensives
Journal of Human Hypertension (2022)
-
Evaluation of Intra-Renal Stiffness in Patients with Primary Aldosteronism
High Blood Pressure & Cardiovascular Prevention (2022)