Abstract
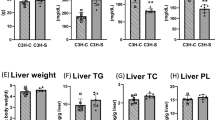
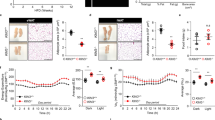
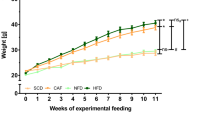
Cholesteryl ester transfer protein (CETP) mediates a step in reverse cholesterol transport, which channels cholesterol from peripheral tissues back to the liver. Mice and rats are CETP-deficient species, which assumedly contribute to rodent atherosclerosis resistance. Both pro- and anti-atherogenic effects have been shown in studies of CETP-transgenic rodent models thus far. As the results of pharmacological studies of CETP modification are largely controversial in humans, further knowledge about the impact of CETP on atherogenic phenotypes is required to evaluate its clinical utility for the prevention of cardiovascular and other organ damage associated with metabolic syndrome. Therefore, we newly generated a human CETP-transgenic (Tg[hCETP]) strain on the genetic background of spontaneously hypertensive rats (SHRs), which are characterized by the spontaneous occurrence of hypertension and insulin resistance. This allowed us to assess the in vivo role of CETP on cardiometabolic phenotypes in combination with hypertension. In Tg[hCETP] SHRs fed normal rat chow, systolic blood pressure was markedly elevated by 20–37 mmHg throughout the study period, and the development of fatty liver was accelerated with appreciable changes in the plasma lipid profile (HDL cholesterol reduction and triglyceride elevation). These phenotypic changes are in accordance with the assumption of proatherogenic effects inducible by the overexpression of CETP. However, with plasma LDL cholesterol levels concomitantly reduced, no apparent progression of atherosclerosis was detected in either the aorta or coronary arteries of Tg[hCETP] SHRs fed a high-fat, high-cholesterol diet. Our data provide new insight into the multifaceted regulation of cardiometabolic phenotypes via the modification of CETP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Microarray data have been deposited at the ArrayExpress Archive of Functional Genomics Data, which is hosted by the EMBL-EBI, under accession number [E-MTAB-7861].
References
Yamashita S, Ruscica M, Macchi C, Corsini A, Matsuzawa Y, Sirtori CR. Cholesteryl ester transfer protein: an enigmatic pharmacology-Antagonists and agonists. Atherosclerosis. 2018;278:286–98.
Maruyama T, Sakai N, Ishigami M, Hirano K, Arai T, Okada S, et al. Prevalence and phenotypic spectrum of cholesteryl ester transfer protein gene mutations in Japanese hyperalphalipoproteinemia. Atherosclerosis. 2003;166:177–85.
Nomura A, Won HH, Khera AV, Takeuchi F, Ito K, McCarthy S, et al. Protein-truncating variants at the cholesteryl ester transfer protein gene and risk for coronary heart disease. Circ Res. 2017;121:81–88.
Blauw LL, Li-Gao R, Noordam R, de Mutsert R, Trompet S, Berbée JFP, et al. CETP (cholesteryl ester transfer protein) concentration: a genome-wide association study followed by mendelian randomization on coronary artery disease. Circ Genom Precis Med. 2018;11:e002034.
Hoffmann TJ, Theusch E, Haldar T, Ranatunga DK, Jorgenson E, Medina MW, et al. Genome-wide characterization of shared and distinct genetic components that influence blood lipid levels in ethnically diverse human populations. Am J Hum Genet. 2013;92:904–16.
Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NG, Jansen H, et al. Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J Am Coll Cardiol. 2017;69:823–36.
Kraja AT, Vaidya D, Pankow JS, Goodarzi MO, Assimes TL, Kullo IJ, et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011;60:1329–39.
Armitage J, Holmes MV, Preiss D. Cholesteryl ester transfer protein inhibition for preventing cardiovascular events: JACC review topic of the week. J Am Coll Cardiol. 2019;73:477–87.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M. ILLUMINATE Investigators et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl J Med. 2007;357:2109–22.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–42.
Buckley MM, Goa KL, Price AH, Brogden RN. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989;37:761–800.
Herrera VL, Makrides SC, Xie HX, Adari H, Krauss RM, Ryan US, et al. Spontaneous combined hyperlipidemia, coronary heart disease and decreased survival in Dahl salt-sensitive hypertensive rats transgenic for human cholesteryl ester transfer protein. Nat Med. 1999;5:1383–9.
Zak Z, Lagrost L, Gautier T, Masson D, Deckert V, Duverneuil L, et al. Expression of simian CETP in normolipidemic Fisher rats has a profound effect on large sized apoE-containing HDL. J Lipid Res. 2002;43:2164–71.
Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–7.
Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, et al. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991;266:10796–801.
Marotti KR, Castle CK, Boyle TP, Lin AH, Murray RW, Melchior GW. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 1993;364:73–75.
Grass DS, Saini U, Felkner RH, Wallace RE, Lago WJ, Young SG. Transgenic mice expressing both human apolipoprotein B and human CETP have a lipoprotein cholesterol distribution similar to that of normolipidemic humans. J Lipid Res. 1995;36:1082–91.
Guyard-Dangremont V, Desrumaux C, Gambert P, Lallemant C, Lagrost L. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:517–25.
Sugano M, Makino N, Sawada S, Otsuka S, Watanabe M, Okamoto H, et al. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J Biol Chem. 1998;273:5033–6.
Hayek T, Masucci-Magoulas L, Jiang X, Walsh A, Rubin E, Breslow JL, et al. Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J Clin Invest. 1995;96:2071–4.
Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–93.
Sato T, Nara Y, Kato Y, Yamori Y. Effects of high-calorie diet on blood pressure and sodium retention in spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Diabet Complic. 1995;9:220–3.
Orbetzova V, Kiprov D, Puchlev A. The action of arterial hypertension on lipid and lipoprotein metabolism. II. Qualitative and quantitative alterations of blood serum, liver and aortic lipids and lipoproteins in Okamoto-Aoki rats with spontaneous hypertension. Cor Vasa. 1976;18:221–32.
Yamori Y, Horie R, Nara Y, Kihara M, Ikeda K, Mano M, et al. Dietary prevention of hypertension in animal models and its applicability to human. Ann Clin Res. 1984;16:28–31.
Yamamoto H, Kanno K, Ikuta T, Arihiro K, Sugiyama A, Kishikawa N, et al. Enhancing hepatic fibrosis in spontaneously hypertensive rats fed a choline-deficient diet: a follow-up report on long-term effects of oxidative stress in non-alcoholic fatty liver disease. J Hepatobiliary Pancreat Sci. 2016;23:260–9.
Svoboda DS, Kawaja MD. Changes in hepatic protein expression in spontaneously hypertensive rats suggest early stages of non-alcoholic fatty liver disease. J Proteom. 2012;75:1752–63.
Shimamoto K, Ura N. Mechanisms of insulin resistance in hypertensive rats. Clin Exp Hypertens. 2006;28:543–52.
Carty CL, Bhattacharjee S, Haessler J, Cheng I, Hindorff LA, Aroda V, et al. Analysis of metabolic syndrome components in >15 000 african americans identifies pleiotropic variants: results from the population architecture using genomics and epidemiology study. Circ Cardiovasc Genet. 2014;7:505–13.
Ogami K, Hadzopoulou-Cladaras M, Cladaras C, Zannis VI. Promoter elements and factors required for hepatic and intestinal transcription of the human ApoCIII gene. J Biol Chem. 1990;265:9808–15.
Kato N, Nabika T, Liang YQ, Mashimo T, Inomata H, Watanabe T, et al. Isolation of a chromosome 1 region affecting blood pressure and vascular disease traits in the stroke-prone rat model. Hypertension. 2003;42:1191–7.
Akiyama K, Liang YQ, Isono M, Kato N. Investigation of functional genes at homologous loci identified based on genome-wide association studies of blood lipids via high-fat diet intervention in rats using an in vivo approach. J Atheroscler Thromb. 2015;22:455–80.
Tzotzas T, Desrumaux C, Lagrost L. Plasma phospholipid transfer protein (PLTP): review of an emerging cardiometabolic risk factor. Obes Rev. 2009;10:403–11.
Chowaniec Z, Skoczyńska A. Plasma lipid transfer proteins: the role of PLTP and CETP in atherogenesis. Adv Clin Exp Med. 2018;27:429–36.
Vergeer M, Stroes ES. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am J Cardiol. 2009;104:32E–38E.
Tall AR. Plasma high density lipoproteins: therapeutic targeting and links to atherogenic inflammation. Atherosclerosis. 2018;276:39–43.
Li Z, Wang Y, van der Sluis RJ, van der Hoorn JW, Princen HM, Van Eck M, et al. Niacin reduces plasma CETP levels by diminishing liver macrophage content in CETP transgenic mice. Biochem Pharm. 2012;84:821–9.
Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018;17:130.
Tomiyama H, Shiina K, Matsumoto-Nakano C, Ninomiya T, Komatsu S, Kimura K, et al. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc. 2017;6:e005729.
Matsunaga T, Hokari S, Koyama I, Harada T, Komoda T. NF-kappa B activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem Biophys Res Commun. 2003;303:313–9.
Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–708.
Kim JY, Kim SM, Kim SJ, Lee EY, Kim JR, Cho KH. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int J Mol Med. 2017;39:889–99.
Castaño G, Más R, Fernández JC, Illnait J, Fernández L, Alvarez E. Effects of policosanol in older patients with type II hypercholesterolemia and high coronary risk. J Gerontol A Biol Sci Med Sci. 2001;56:M186–192.
Carvalho LS, Virginio VW, Panzoldo NB, Figueiredo VN, Santos SN, Modolo RG, et al. Elevated CETP activity during acute phase of myocardial infarction is independently associated with endothelial dysfunction and adverse clinical outcome. Atherosclerosis. 2014;237:777–83.
Simic B, Mocharla P, Crucet M, Osto E, Kratzer A, Stivala S, et al. Anacetrapib, but not evacetrapib, impairs endothelial function in CETP-transgenic mice in spite of marked HDL-C increase. Atherosclerosis. 2017;257:186–94.
Ioannou GN. Beyond obesity: is cholesterol-induced liver injury the cause of non-alcoholic steatohepatitis? J Gastroenterol Hepatol. 2012;27:1412–4.
Haas JT, Staels B. Cholesteryl-ester transfer protein (CETP): a Kupffer cell marker linking hepatic inflammation with atherogenic dyslipidemia? Hepatology. 2015;62:1659–61.
Blake WL, Ulrich RG, Marotti KR, Melchior GW. The development of fatty liver is accelerated in transgenic mice expressing cynomolgus monkey cholesteryl ester transfer protein. Biochem Biophys Res Commun. 1994;205:1257–63.
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1–8.
Herrera VL, Tsikoudakis A, Didishvili T, Ponce LR, Bagamasbad P, Gantz D, et al. Analysis of gender-specific atherosclerosis susceptibility in transgenic[hCETP]25DS rat model. Atherosclerosis. 2004;177:9–18.
Marotti KR, Castle CK, Murray RW, Rehberg EF, Polites HG, Melchior GW. The role of cholesteryl ester transfer protein in primate apolipoprotein A-I metabolism. Insights from studies with transgenic mice. Arterioscler Thromb. 1992;12:736–44.
Mashimo T, Ogawa H, Cui ZH, Harada Y, Kawakami K, Masuda J, et al. Comprehensive QTL analysis of serum cholesterol levels before and after a high-cholesterol diet in SHRSP. Physiol Genom. 2007;30:95–101.
Tomita T, Shirasaki Y, Yonekura I, Hayashi E. Sex differences in enzyme activities relating to cholesteryl ester metabolism in aorta and liver from SHRSP, SHR, and WKR during aging. Jpn Heart J. 1978;19:669–70.
Herrera VL, Ponce LR, Ruiz-Opazo N. Genome-wide scan for interacting loci affecting human cholesteryl ester transfer protein-induced hypercholesterolemia in transgenic human cholesteryl ester transfer protein F2-intercross rats. J Hypertens. 2007;25:1608–12.
Zhao Y, Yang Y, Xing R, Cui X, Xiao Y, Xie L, et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis. 2018;271:26–35.
Wei S, Zhang Y, Su L, He K, Wang Q, Zhang Y, et al. Apolipoprotein E-deficient rats develop atherosclerotic plaques in partially ligated carotid arteries. Atherosclerosis. 2015;243:589–92.
Sithu SD, Malovichko MV, Riggs KA, Wickramasinghe NS, Winner MG, Agarwal A, et al. Atherogenesis and metabolic dysregulation in LDL receptor-knockout rats. JCI Insight. 2017;2:86442.
Yamashita S, Masuda D, Ohama T, Arai H, Bujo H, Kagimura T, et al. Rationale and design of the PROSPECTIVE trial: probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease. J Atheroscler Thromb. 2016;23:746–56.
Acknowledgements
We are grateful to Koichi Akiyama of the Research Institute, National Center for Global Health and Medicine, for his technical assistance with DNA analysis. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This study was supported by a grant from the National Center for Global Health and Medicine and JSPS KAKENHI Grant no. 26290067.
Author information
Authors and Affiliations
Contributions
NK conceived and designed the study and wrote the manuscript. Y-QL, MI, and TO performed the experimental analyses. FT performed the data analyses. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, YQ., Isono, M., Okamura, T. et al. Alterations of lipid metabolism, blood pressure and fatty liver in spontaneously hypertensive rats transgenic for human cholesteryl ester transfer protein. Hypertens Res 43, 655–666 (2020). https://doi.org/10.1038/s41440-020-0401-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0401-9