Abstract
Although beta blockers have been used as initial therapy for ischemic heart diseases and heart failure, the beneficial effects of beta blockers are controversial compared with other antihypertensive agents as initial therapy for hypertension without compelling indications. Moreover, atenolol has been most commonly used with beta blockers. The objective of the present systematic review associated with the Japanese Society of Hypertension (JSH) 2019 Hypertension Guideline (Clinical Question 6) was to assess the outcomes (cardiocerebrovascular mortality, total cause mortality, hypotension, bradycardia, other adverse effects, and changes in systolic blood pressure (SBP)) of currently used carvedilol and bisoprolol as initial therapy for adult hypertension without compelling indications. Two independent systematic reviewers searched randomized controlled trials (RCTs) up to October 2017 in the Cochrane Hypertension Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, EMBASE Ovid, and ClinicalTrials.gov. Finally, eight RCTs with 2494 participants were identified to meet our inclusion criteria. There were no RCTs in which cardiocerebrovascular mortality, total cause mortality, hypotension, and bradycardia were assessed between carvedilol or bisoprolol and placebo. SBP-lowering effects were significantly increased for bisoprolol compared with placebo. Here, 50 mg carvedilol significantly reduced SBP compared with placebo, whereas 12.5 mg or 25 mg did not. Regarding adverse effects, no differences were noted between carvedilol and placebo (two RCTs, 286 participants, moderate certainly evidence). In conclusion, current evidence does not support carvedilol or bisoprolol as first-line therapy for adult hypertension without compelling indications.
Similar content being viewed by others
Introduction
Description of the condition
Hypertension is one of the most common causes of cardiovascular diseases, and hypertension treatments significantly impact cardiovascular mortality [1]. Current available major classes of antihypertensive drugs include calcium channel blockers, renin–angiotensin system inhibitors (angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers), beta blockers, and diuretics.
Description of the intervention
Beta blockers have been used as initial therapy for hypertension since the late 1960s because abnormal sympathoexcitation is important in the pathophysiology of hypertension. A systematic review examined the mortality and morbidity outcomes of different classes of initial antihypertensive drugs in patients with hypertension and revealed that beta blockers significantly reduced stroke and cardiovascular events but not all-cause mortality and congestive heart disease [1]. The results of clinical outcomes with beta blockers were inferior compared with low-dose thiazides, ACE inhibitors, and calcium channel blockers [1]. Regarding the blood pressure (BP)-lowering effect, two systematic reviews have assessed the effects of beta1-selective blockers or dual alpha and beta blockers on BP control. A Cochrane review of the BP-lowering efficacy of dual alpha and beta blockers for primary hypertension revealed low quality evidence suggesting that dual alpha and beta blockers reduce BP by an average of −6 / −4 mmHg in patients with mild to moderate hypertension. In addition, the BP-lowering effects were less than those for beta1-selective blockers [2]. Another Cochrane review of blood pressure-lowering efficacy of beta1-selective beta blockers for primary hypertension revealed low quality evidence that suggested that beta1-selective blockers reduced BP by an average of −10 / −8 mmHg compared with placebo. In addition, beta1-selective blockers reduced BP by a greater magnitude compared with dual alpha and beta blockers in patients with mild to moderate hypertension [3].
How the intervention might work
Among beta blockers, propranolol exhibits affinity for beta1 and beta2 receptors and is thus classified as non-selective beta blocker. Atenolol and bisoprolol preferentially interacts with beta1 compared with beta2 receptors and are referred to as selective beta blockers. Carvedilol exhibits affinity for beta1 and alpha receptors and is classified as dual alpha and beta blocker.
Why this review is important
Previous systematic reviews have indicated that beta blockers are not the recommended class of drugs to use as initial antihypertensive therapy. However, regarding pharmacological mechanisms, beta blockers with different affinities have different effects on BP reduction. Moreover, atenolol was the most commonly used beta blocker reported in previous studies. In Japan, carvedilol and bisoprolol are currently the most commonly used drugs. The present systematic review could provide beneficial evidence of carvedilol and bisoprolol in hypertensive adults without compelling indications.
Objectives
The objective of the present systematic review associated with Japanese Society of Hypertension (JSH) 2019 Hypertension Guideline (Clinical Question 6) was to assess the outcomes (cardiocerebrovascular mortality, total cause mortality, hypotension, bradycardia, other adverse effects, and changes in systolic BP (SBP)) of carvedilol and bisoprolol as initial therapy for adult hypertension without compelling indications.
Methods
Study design for this review
The present review is a systematic review and meta-analysis.
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT) were assessed.
Types of participants
Males and non-pregnant females aged 18 years and older with hypertension as defined by cut-off points operating at the time of the study were under consideration for inclusion in this study.
Types of interventions
The treatment group must have received carvedilol or bisoprolol as monotherapy or as an initial drug. The control group includes placebo, no treatment, or another antihypertensive drug (including a different beta blocker).
Types of outcome measures
Cardiocerebrovascular mortality, total cause mortality, hypotension, bradycardia, other adverse effects, and changes in SBP served as outcomes.
Search methods
Two independent systematic reviewers of the Japanese Society of Hypertension searched the following databases for randomized controlled trials up to October 2017: The Cochrane Hypertension Specialized Register via the Cochrane Register of Studies (CSR-Web) (searched 18 October 2017); the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CSR-Web) (searched 18 October 2017); MEDLINE Ovid (from 1946 to 2017), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In-Process & Other Non-Indexed Citations (searched 18 October 2017); Embase Ovid (from 1974 to 2017) (searched 18 October 2017); and ClinicalTrials.gov (www.clinicaltrials.gov) (searched 18 October 2017). We checked reference lists of relevant reviews, guidelines, and references lists of studies potentially eligible for inclusion in this review.
Subject strategies for databases on the search strategy designed for MEDLINE were modeled (Supplementary Information). Where appropriate, subject strategies were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions).
Data collections and analysis
Selection of studies
Initial searches of all the databases were performed to identify citations with potential relevance. The initial screening of these abstracts excluded those studies with titles, abstracts or both that were clearly irrelevant. The full texts of the remaining articles were retrieved.
Data extraction and management
Two systematic reviewers independently extracted data using a standard form and crosschecked the data. A second person confirmed all numeral calculations and graphic interpolations.
Assessment of risk of bias in included studies
We also assessed the risk of six biases by addressing selection bias (randomization and concealment), performance bias (blinding), detection bias (blinding), and attrition bias (ITT and incomplete outcome data) as described in the Cochrane Handbook for Systematic Reviews of Interventions. For each included study, we described what the study authors reported for each domain and then made a decision related to the risk of bias for the domain by assigning a judgment of the certainty of the evidence as high (if we are confident that the true effect is close to that of the estimate of effect), moderate (if the true effect is likely to be close to the estimate of effect), low (if the true effect may be substantially different from the estimate of effect), and very low (if we are very uncertain about the estimate of effect). The data extracted for each study included methods, including means of assigning participants to trials interventions, blinding of those receiving and providing care and outcome assessors, losses to follow-up and how they were handled, and length of trial follow-up; participant characteristics, including gender, ethnicity, and comorbid conditions; interventions, including type and dose of carvedilol, bisoprolol, and other medications used; outcome measures, including morbidity and mortality endpoints and adverse events.
Measures of treatment effect
We combined data for changes in BP using mean difference. We analyzed dropout due to side effects using risk ratios, which were expressed as risk ratios with 95% confidence intervals, and conducted random-effects with inverse variance weighting for all the analysis as appropriate.
Assessment of heterogeneity
Heterogeneity of treatment effects between the trials was assessed using a standard Chi [2] statistic for heterogeneity. In addition, we used I2 statistics to describe the percentage of between study variability.
Data syntheses
We performed data synthesis and analysis using the Cochrane Review manager software RevMan 5.3.5. Data for changes in SBP were combined using mean differences. Dropouts due to side effects were analyzed using risk ratios.
Main results
Description of studies
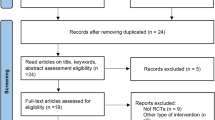
The PRISMA flow diagram for this systematic review is presented in Fig. 1 [4]. We obtained 906 records from the search conducted on October 18, 2017. None of the “ongoing” studies from Clinicaltrials.gov and WHO International Clinical Trials Registry Platform were included in this analysis. We screened these records, and excluded 890 studies that were not relevant to the analysis. Sixteen full-text articles were assessed for eligibility, and eight studies were excluded because they were not relevant to the analysis. From these searches, we identified eight RCTs with 2494 participants that meet our inclusion criteria: McPhillips 1988 [5], Asmar 1991 [6], Broekman 1992 [7], Davidov 1994 [8], Frishman 1995 [9], Deary 2001 [10], Deary 2002 [11], and Weber 2006 [12]. These RCTs were conducted between 1980s and 2000s and compared carvedilol at 12.5 mg (one RCT, 10 participants) [5], 25 mg (two RCTs, 85 participants) [5, 12], and 50 mg (two RCTs, 77 participants) [5, 12] or bisoprolol at 5 mg (five RCTs, 2187 participants) [7,8,9,10,11], 10 mg (two RCTs, 74 participants) [6, 8], and 20 mg (one RCT, 61 participants) [8] with a placebo. Seven RCTs recruited participants of both sexes, and one RCT exclusively recruited males [7]. All RCTs included similar mean aged participants (i.e., approximately 50 years old). All RCTs conducted in industrialized country provided information on race or ethnicity. We have described the characteristics and main features of these eight RCTs included in the present systematic review in Supplementary Table.
Risk of bias included studies
The risk of bias in included studies is summarized in Table 1.
Selection bias (randomization and concealment)
Because details regarding selection bias (randomization and concealment) were poorly reported in many of the included studies, it was difficult to judge the potential for selection bias. In the three RCTs, the risk bias of randomization was low, and we did not identify sufficient information for risk bias of randomization in the other five RCTs. In addition, we did not identify sufficient information for risk bias of concealment in all RCTs in the present systematic review.
Blinding (performance and detection bias)
In the six RCTs in the present systematic review, the blinding of performance bias is low because placebo was used. However, in the two RCTs, the blinding of performance bias is high because the physician determined the best treatment for hypertension [10, 11]. Regarding the blinding of the detection bias, the ability of beta blockers to reduce heart rate is well known. The assessor could detect the difference in heart rate when measuring BP. Using an automated machine to measure BP would mitigate this risk of detection bias. However, this procedure was performed in four RCTs in this systematic review [5, 8, 9, 12]. Therefore, the risk of performance and detection bias is high.
Attrition bias (ITT and incomplete outcome data)
Most of the RCTs reported the method for handling dropouts, and the dropout rates were low. Moreover, most of the RCTs used an ITT analysis. Therefore, we judged that the risk of attrition bias in this systematic review was low.
Selective reporting
All studies reported SBP and DBP as outcomes of the participants. Only one study (McPhillips 1988) did not report data for heart rate [5]. Withdrawal due to adverse effects is an importance outcome in clinical trials. Only three studies reported useful data on withdrawal due to adverse effects. The risk of reporting bias was high because most of the studies failed to report withdrawal due to adverse effects.
Other potential sources of bias
Funnel plots of the pooled data revealed a paucity of small and less effective studies with positive outliers, and the asymmetry of funnel plots indicated the high potential for publication bias in the SBP-lowering efficacy of bisoprolol and carvedilol (Fig. 2a) and withdrawal due to adverse effects of carvedilol (Fig. 2b). Given that we only included eight RCTs in this systematic review, which is an insufficient number for funnel plot assessment, it was not possible to prove that these trials were flawed and that they added to the suspicion of a high risk of bias.
Effects of interventions
There were no RCTs in which cardiocerebrovascular mortality, total cause mortality, hypotension, and bradycardia were assessed between carvedilol or bisoprolol and placebo.
SBP-lowering efficacy of carvedilol
We included two RCTs that examined the SBP-lowering efficacy of 12.5 or 50 mg carvedilol once daily in 34 mild to moderate hypertensive patients [5, 12]. The SBP-lowering effect was increased for carvedilol at 50 mg (mean difference −8.33, 95% CI −12.00 to −3.77, I2 = 0%; very low certainty evidence) but not 12.5 mg or 25 mg (Fig. 3).
SBP-lowering efficacy of bisoprolol
Two of the included RCTs employed a parallel design [8, 9], and four RCTs employed a cross-over design [6, 7, 10, 11]. The SBP-lowering effect was increased for bisoprolol compared with placebo (5 mg; mean difference −5.75, 95% CI −6.38 to −5.13, I2 = 93%; very low certainty evidence, 10 mg; mean difference −0.96, 95% CI −11.06 to −2.56, I2 = 35%; very low certainty evidence, 20 mg; mean difference −7.60, 95% CI −12.64 to −2.56; very low certainty evidence) (Fig. 4). Significant extreme statistical heterogeneity was noted between trials comparing 5 mg bisoprolol to placebo (χ2 = 54.59, degrees of freedom = 4, P < 0.000001, I2 = 93%).
Withdrawal due to adverse effects
Regarding adverse effect, no differences were noted between carvedilol and placebo (2 RCTSs, 286 participants, I2 = 0%; moderate certainty evidence).
Discussion
Summary of main results
Eight RCTs in this systematic review revealed very low certainty that the SBP-lowering effect of bisoprolol was increased compared with placebo (5 mg; mean difference −5.75, 95% CI −6.38 to −5.13, I2 = 93%; very low certainty evidence, 10 mg; mean difference −0.96, 95% CI −11.06 to −2.56, I2 = 35%; very low certainty evidence, 20 mg; mean difference −7.60, 95% CI −12.64 to −2.56; very low certainty evidence). In addition, 50 mg carvedilol significantly reduced blood pressure compared with placebo (mean difference −8.33, 95% CI −12.00 to −3.77, I2 = 0%; very low certainty evidence) but not 12.5 mg or 25 mg carvedilol. Regarding adverse effects, no differences were noted between carvedilol and placebo (2 RCTs, 286 participants, I2 = 0%; moderate certainly evidence).
Overall completeness and applicability of evidence
The present systematic review provides the most current evidence for the SBP-lowering efficacy of carvedilol or bisoprolol. The RCTs included in the present systematic review met criteria regarding population, goal of the studies and outcome measured. Our search strategy was comprehensive and thorough. Therefore, we are confident that the present systematic review represents the best evidence available for SBP-lowering efficacy of carvedilol and bisoprolol.
Quality of evidence
The present systematic review included eight RCTs examining carvedilol and bisoprolol in 2494 adult hypertensive patients. The sample size of RCTs with carvedilol was relatively insufficient to allow a robust conclusion (381 participants).
Half of the RCTs in the present systematic review did not use automated BP machines, which potentially mitigate the risk of detection bias caused by loss of blinding. Thus, the risk of detection bias remains high, and the quality of evidence was downgrade by one level.
Potential biases in the review process
The rigidity of the inclusion criteria minimized the potential of bias during the selection process. Study selection was exclusively based on methodology. The responsibility of the review authors was to determine whether the methodology met the inclusion criteria. No other part of the study played a role in the decision. These strict inclusion criteria ensured that included RCTs were all performed in a manner that minimized the risk of bias during the process.
Agreements and disagreements with other studies or reviews
In the previous systematic reviews of the effects of beta blockers on morbidity and mortality endpoints in adults with hypertension (13 RCTs, 40,245 participants), no differences in all-cause mortality were noted between beta blockers and placebo, diuretics or renin–angiotensin system inhibitors; however, the effects of beta blockers were increased compared with calcium channel blockers [1]. The evidence on mortality was of moderate certainty for all comparisons, and most outcome RCTs on beta blockers as initial therapy for hypertension exhibit a high risk of bias. In conclusion, these previous reviews suggested that the effects of beta blockers are inferior to those of other antihypertensive drugs. Three-quarters of these RCTs assessed atenolol, and the effects of carvedilol or bisoprolol were not assessed. No current evidence is available to assess the effects of carvedilol and bisoprolol on cardiocerebrovascular mortality and all-cause mortality in adult patients without compelling indications.
In the previous systematic review of the dose-related effects of various types of dual alpha and beta blockers on SBP and DBP versus placebo in patients with primary hypertension (eight RCTs, 1493 participants), the estimate of BP-lowering effect (SBP / DBP) were −4 (95% CI −6 to −2) / −3 (95% CI −4 to −2) for carvedilol (>1000 participants) with low quality of evidence [2]. However, this systematic review involved two large unpublished studies with carvedilol, which were excluded in the present systematic review. Similar evidence on the SBP-lowering effects of bisoprolol compared with previous systematic reviews with various beta1-selective blockers was revealed [3]. However, the certainty of evidence was very low in the present systematic review.
Regarding adverse effects, the present systematic review revealed similar and moderate quality evidence with carvedilol compared with previous reviews. However, evidence demonstrating the adverse effects of bisoprolol is not available.
Conclusion
Most outcome RCTs on carvedilol or bisoprolol as initial therapy for hypertensive adults without compelling indications exhibit a high risk of bias and very low certainty. A low quality of evidence means that future research is very likely to have an important impact on our confidence of the estimate of the effect. Current evidence does not support carvedilol or bisoprolol as first-line therapy for adult hypertension without compelling indications.
References
Wright JM, Musini VM. First-line drugs for hypertension. Cochrane Database Syst Rev. 2009; CD001841.
Wong GWK, Laugerotte A, Wright JM. Blood pressure lowering efficacy of dual alpha and beta blockers for primary hypertension (Review). Cochrane Database Syst Rev. 2015; CD007449.
Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension (Review). Cochrane Database Syst Rev. 2017; CD002003.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Pre-ferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;89:873–80.
McPhillips JJ, Schwemer GT, Scott DI, Zinny M, Patterson D. Effects of carvedilol on blood pressure in patients with mild to moderate hypertension. A dose response study. Drugs. 1988;36(supple6):82–91.
Asmar RG, Kerihuel JC, Girerd VJ, Safar ME. Effect of bisoprolol on blood pressure and arterial hemodynamics in systemic hypertension. Am J Cardiol. 1991;68:61–64.
Broekman CP, Haensel SM, Van de Ven LL, Slob AK. Bisoprolol and hypertension: effects on sexual functioning in men. J Sex Marital Ther. 1992;18:325–31.
Davidov ME, Singh SP, Vlachakis ND, Blumenthal JB, Simon JS, Bryzinski BS. et al. Bisoprolol, a once-a-day beta-blocking agent for patients with mild to moderate hypertension. Clin Cardiol. 1994;17:263–8.
Frishman WH, Burris JF, Mroczek WJ, Weir MR, Alemayehu D, Simon JS, et al. First-line therapy option with low-dose bisoprolol fumarate and low-dose hydrochlorothiazide in patients with stage I and stage II systemic hypertension. J Clin Pharmacol. 1995;35:182–8.
Deary AJ, Schumann AL, Murfet H, Haydock SF, Foo RS, Brown MJDouble-blind. placebo-controlled crossover comparison of five classes of antihypertensive drugs. J Hypertens. 2001;20:771–7.
Deary AJ, Schumann AL, Murfet H, Haydock S, Foo RS, Brown MJ. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension. Clin Sci. 2002;103:493–9.
Weber MA, Barkris GL, Tarka EA, Iyengar M, Fleck R, Sica DA. Efficacy of a once-daily formulation of carvedilol for the treatment of hypertension. J Clin Hypertens. 2006;8:840–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Kishi, T., Fujii, E. Carvedilol and bisoprolol as initial therapy for adult hypertension without compelling indications. Hypertens Res 42, 496–503 (2019). https://doi.org/10.1038/s41440-018-0174-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0174-6
Keywords
This article is cited by
-
Drug release profiles of Atenolol and Benidipine from pH-responsive polymeric hydrogel matrix
Chemical Papers (2023)
-
The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019)
Hypertension Research (2019)