Abstract
Clinical implication of a high ankle-brachial index (ABI) is not well known. Based on our previous study, we suspected that body composition may be a determinant of a high ABI and may consequently modulate the clinical significance of a high ABI. Datasets of two studies with independent cohorts, the anti-aging study cohort (n = 1765) and the Nagahama study cohort (n = 8,039), were analyzed in this study, in which appendicular muscle mass was measured by computed tomography and bioelectrical impedance analysis, respectively. Brachial and ankle blood pressures were measured using a cuff-oscillometric method. In the anti-aging study cohort, thigh muscle area (β = 0.387, p < 0.001), but not fat area, showed a strong positive association with the ABI independent of the body mass index (p = 0.662) and other possible covariates, including systolic brachial blood pressure (p = 0.054), carotid hypertrophy (p = 0.559), and arterial stiffness (β = 0.102, p = 0.001). This positive association was replicated in the Nagahama cohort. When the subjects were subdivided by the 75th percentiles of the ABI and appendicular muscle mass, multinomial logistic regression analysis identified insulin resistance as an independent determinant of an elevated ABI in subjects with normal muscle mass (coefficient = 0.134, p = 0.010), whereas insulin resistance was inversely associated with an elevated ABI in subjects with high muscle mass (coefficient = −0.268, p = 0.001). Appendicular muscle mass was a strong determinant of the ABI. The clinical background, particularly insulin resistance, of individuals with an elevated ABI may differ based on the amount of muscle mass.
Similar content being viewed by others
Introduction
A low ankle-brachial index (ABI), a ratio of ankle systolic blood pressure (SBP) to brachial SBP, is associated with cardiovascular risk factors [1] as well as the incidence of cardiovascular diseases and mortality [2]. An ABI of 0.9 is usually adopted as a lower cut-off point to discriminate at-risk individuals; however, a recent large-scale meta-analysis by the Ankle Brachial Index Collaboration revealed increased risks for total and cardiovascular morbidity and mortality even in individuals with a low-normal ABI (0.9 ≤ ABI < 1.0) [3]. In contrast, the clinical implication of a high ABI is less understood. Several cross-sectional studies have reported that a high ABI, usually defined as an ABI greater than 1.4, was associated with left ventricular hypertrophy [4], increased coronary artery calcium scores [5], and chronic kidney disease [6] in general [4, 5] and high-risk populations [6]. Furthermore, a high ABI has been suggested to have prognostic significance for the incidence of coronary artery disease [6,7,8] as well as cardiovascular [9] and total mortality [9, 10], although the results were not always consistent across studies. The inconsistencies may be due to different population characteristics and a particularly low frequency of individuals with a high ABI (1.1 to 4.9%), although the Strong Heart Study with high-risk individuals showed a greater frequency (9.2%) of a high ABI [9].
We previously reported that muscle mass, but not fat mass, in the lower extremities measured directly from computed tomography (CT) images was significantly and positively associated with the ABI in a general population [11]. This finding suggested that in addition to medial arterial calcification, which is known to increase ankle BP [12], incompressibility of the tibial artery due to high appendicular muscle mass may be a cause of a spuriously high ABI. Consistent characteristics among high ABI populations in previous studies, namely, a large body mass index (BMI) [5, 6, 8, 10, 13,14,15] and a higher frequency of men [4, 6,7,8, 10, 13, 14], support the involvement of lower extremity composition in increasing the ABI because body weight and male sex are strong determinants of skeletal muscle mass. Absence of an association between total arterial compliance and a high ABI [14] also supports the hypothesis.
In addition to the muscle involvement hypothesis, other common characteristics of individuals with a high ABI, namely, non-smoking [5, 6, 8, 10, 13, 14], relatively low brachial BP [5, 7, 9, 10, 13, 14], and good plasma lipid profiles [4, 5, 7, 10, 14], further support the hypothesis that a high ABI may not always represent poor arterial properties, and the clinical implication of a high ABI may differ according to the amount of appendicular muscle mass.
Here, we performed an extended analysis of our preceding study that first reported a positive relationship between thigh muscle mass and the ABI [11] and performed an additional analysis in a large cohort to test these hypotheses.
Methods
Study participants
The present study incorporated datasets from two Japanese general populations.
The Shimanami Health Promoting Program (J-SHIPP study) is a longitudinal study conducted by Ehime University Graduate School of Medicine evaluating factors related to cardiovascular disease (CVD), dementia, and death among several cohorts from the general population in Ehime Prefecture, Japan. Here, we analyzed a dataset from the Anti-Aging Study Cohort (AASC) [16,17,18,19]. The AASC includes apparently healthy, middle-aged to elderly participants in the medical check-up program at the Ehime University Hospital Anti-aging Center. This medical check-up program was offered to general residents of Ehime Prefecture and was specifically designed to evaluate age-related disorders, such as atherosclerosis, CVD, physical dysfunction, and cognitive impairment. From a total of 2034 individuals who participated from February 2006 to December 2016, we analyzed a dataset of participants for whom relevant clinical data, including thigh muscles mass, were available and those with a normal ABI (>0.9) in both legs (n = 1765). Because an ABI less than 0.9 is usually used as a cut-off point to discriminate potential peripheral arterial disease, we excluded individuals with an ABI less than 0.9 in this study to clarify the effect of appendicular muscle mass on the ABI.
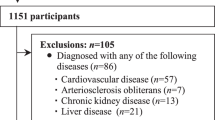
The Nagahama Prospective Cohort for Comprehensive Human Bioscience (the Nagahama Study) is a community-based prospective cohort study in which participants were recruited between 2008 and 2010 from 30- to 74-year-old residents in Nagahama City (n = 9764). The participants in the Nagahama cohort were invited to a follow-up assessment that was conducted from 2013 to 2015, 5 years after the baseline evaluations, respectively. Among the 8289 participants in the follow-up investigation, individuals who met the following criteria were excluded from the analysis: pregnant women (n = 24), those with implanted pacemakers (n = 12), those undergoing hemodialysis therapy (n = 5) or insulin therapy (n = 45), those with incomplete or wide deviations in clinical values required for the present study (n = 85), and those with an ABI lower than 0.9 in either leg (n = 79). Details of the Nagahama study participants are described elsewhere [20, 21].
All study procedures were approved by the ethics committee of Ehime University Graduate School of Medicine (the AASC cohort) or that of Kyoto University Graduate School of Medicine and the Nagahama Municipal Review Board (the Nagahama cohort). Written informed consent was obtained from all participants.
Thigh muscle and fat mass measurements (the AASC cohort)
CT of the abdomen and mid-thigh was performed as an optional examination upon request. Cross-sectional areas (CSAs) of the cortical bone and thigh muscle, including the quadriceps, adductors, and hamstrings, were measured from a CT image (LightSpeed VCT; GE Healthcare, Tokyo, Japan) at the midpoint of the lower margin of the femoral condyles and the upper margin of the greater trochanter. The CSA (cm2) was computed using OsiriX software [22] with an attenuation range of 0–1000 Hounsfield units for cortical bone CSA and 0–100 Hounsfield units for muscle CSA. The area of subcutaneous thigh fat was also measured from the same image [total CSA (−200 to 100 Hounsfield units) − muscle CSA − bone CSA (cortical bone, trabecular bone, and bone marrow area)]. CT images were obtained at a minimal slice width of 5 mm.
Estimation of lean mass (the Nagahama cohort)
Appendicular lean mass (ALM) was estimated by bioelectrical impedance analysis (InBody 430, InBody Co., Ltd, Seoul, Korea). A device measures the resistance and reactance of the arms, trunk, and legs separately at three frequencies (5, 50, and 250 kHz) of alternating current of 250 A using a tetrapolar, eight-point tactile electrode system. Total body water was estimated using the sum of five segmental impedances and then fat-free mass was calculated based on the assumption that the hydration of fat-free mass is 73.2%. Lean mass was estimated by subtracting the bone mass calculated using a prediction equation based on dual-energy X-ray absorptiometry (DXA) measurement from the fat-free mass [23, 24]. The skeletal muscle mass index (SMI) was obtained by dividing the ALM by body height squared [25]. The validity and reproducibility of ALM and fat mass measurements by the segmental multiple-frequency BIA (SMF-BIA) method have been reported compared to those of measurements obtained by DXA [23, 24, 26,27,28] and hydrostatic weighing [23], although fat mass estimation in obese subjects may be susceptible to measurement errors [25,26,27,28].
ABI and pulse wave velocity
Brachial and ankle BPs were measured in the supine position. Cuffs were attached to the brachia and ankles, and BPs were measured simultaneously using the cuff-oscillometric method (AASC cohort: PWV/ABI, Omron Healthcare, Co., Ltd, Kyoto, Japan; Nagahama cohort: Vasera-1500, Fukuda Denshi Co., Ltd, Tokyo, Japan). The ABI was calculated as the ratio of ankle SBP to brachial SBP. The mean of the right-side and left-side ABI values was used in the analysis as a representative value.
Pulse volume waveforms were also recorded simultaneously using a plethysmographic sensor connected to the cuffs. Brachial-to-ankle pulse wave velocity (baPWV) was calculated from the time interval between the wave fronts of the brachial and ankle waveforms (∆Tba) by the following formula: baPWV = (La – Lb)/∆Tba, where Lb and La indicate the path lengths from the suprasternal notch to the brachium [Lb = 0.2195 × body height (cm)–2.0734] and to the ankle [La = 0.8129 × body height (cm)–12.328], respectively [29]. The collinearity of baPWV with carotid-to-femoral PWV, a standard measure of arterial stiffness, has been reported elsewhere [30, 31].
Carotid ultrasonography
The intima-media thickness (IMT) of the carotid artery was measured as an index of atherosclerosis. In the AASC cohort, ultrasonography of the common carotid artery was performed using an SSD-3500SV or α10 ultrasonograph (Aloka Co., Ltd, Tokyo, Japan) with a 7.5-MHz probe. After a few minutes of rest in the supine position, optical visualization of the bilateral carotid arteries was obtained with the subject’s head tilted slightly upward in the mid-line position. The IMT of the far wall was measured from B-mode images using built-in computerized software, which simultaneously measured the IMT at three points at 1-cm intervals. Nine IMT measurements of the far wall were obtained at 1-cm intervals proximal to the bulb from the anterior, lateral, and posterior directions. The mean IMT calculated from the nine measurements was used in the analysis. No measurements were taken at the level of a discrete plaque. In the Nagahama cohort, carotid IMT was measured using a Prosound 2 (Aloka), SSD-3500SV (Aloka), or XARIO SSA-660A (Toshiba Medical Systems Corporation, Tochigi, Japan) ultrasonograph with a 7.5-MHz probe. After a few minutes of rest in the supine position, IMT measurements of the far wall were manually (Aloka) or automatically (Toshiba) obtained from B-mode images at 1-cm intervals proximal to the bulb from the lateral direction. The mean IMT calculated from the three readings was used in the analysis.
Basic clinical parameters
The basic clinical parameters used in this study were obtained from the subjects’ personal health records from the baseline examination. Brachial BP was measured in a sitting position after a few minutes of rest using a cuff-oscillometric device (HEM9000-AI; Omron Healthcare). Plasma markers were measured using peripheral blood samples. In the AASC cohort, the low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald equation [total cholesterol − high-density lipoprotein (HDL) cholesterol − triglyceride/5], and in the Nagahama cohort, it was measured directly using a selective solubilization assay (MetaboLead LDL-C; Kyowa Medix, Co., Ltd, Tokyo, Japan). Insulin resistance was assessed using the homeostasis model assessment index for insulin resistance {HOMA-IR: [insulin (μU/ml) × glucose (mg/dl)]/405}. Because blood samples from some of the Nagahama cohort participants were collected under non-fasting (<5 h, 5.7%) or near-fasting (5–11 h, 21.7%) conditions, the elapsed time after the last meal (fasting time) was included in the regression analysis as a covariate. Information regarding medication history and smoking habits was obtained using a structured questionnaire.
Statistical analysis
The values are expressed as the mean ± standard deviation. The quartiles of thigh CSA and the SMI were calculated within sex and then combined to avoid potential sex differences. Group differences in numeric variables were assessed by analysis of variance, and a post hoc analysis was performed using Dunnett’s test. Factors independently associated with subgroups defined by the ABI and SMI were identified using a multinomial logistic regression model. Statistical analyses were performed using commercially available statistical software (JMP ver. 12.2.0; SAS Institute, Cary, NC, USA). Null hypotheses were rejected at a p < 0.05 level of significance.
Results
The clinical characteristics of the study participants in both cohorts are shown in Table 1. The mean age of the AASC participants was slightly higher than that of the Nagahama cohort participants.
Scatter plots of the thigh muscle CSA and ABI in the AASC cohort (Fig. 1a) indicated a significant positive association between these parameters. Because apparent sex differences in the ABI were observed (p < 0.001), particularly in the muscle CSA (p < 0.001), the participants were subdivided into quartiles within sex and then group differences in the ABI were analyzed (Fig. 1b). The results clarified a sex-independent positive association between muscle CSA and the ABI, whereas the ABI in the high-fat CSA subgroup was slightly lower than that in the low-fat CSA subgroup (Fig. 1c). Although BMI was another strong determinant for thigh muscle CSA (men, r = 0.555; women, r = 0.594), multiple linear regression analysis identified muscle CSA (β = 0.387, p < 0.001), but not BMI (p = 0.662), as a strong positive determinant of the ABI independent of possible covariates, including age (β = 0.052, p = 0.080), the Brinkman index (β = −0.065, p = 0.009), systolic BP (β = 0.055, p = 0.054), triglyceride level (β = −0.094, p < 0.001), HDL cholesterol level (β = −0.005, p = 0.847), LDL cholesterol level (β = −0.052, p = 0.023), HOMA-IR (β = −0.060, p = 0.023), baPWV (β = 0.102, p = 0.001), and carotid IMT (β = −0.016, p = 0.559), whereas thigh fat CSA was identified as an inverse determinant (β = −0.253, p < 0.001). When both muscle and fat CSAs were included in the same regression model, muscle CSA (β = 0.348, p < 0.001, variation inflation factor (VIF) = 2.78), but not fat CSA (β = −0.058, p = 0.087, VIF = 2.32), was identified as an independent determinant of the ABI. In these regression analyses, we did not include sex in the model because of moderate collinearity with muscle CSA. However, even in a regression model including sex (women: β = −0.087, p = 0.055, VIF = 4.19), muscle CSA was identified as an independent determinant of the ABI (β = 0.286, p < 0.001, VIF = 4.87).
Association between thigh muscle CSA and the ABI in the AASC cohort. a The scatter plot is depicted according to sex, whereas the correlation coefficient was calculated using the total population. b, c Quartiles of thigh muscle CSA and fat CSA were defined according to sex and then combined to avoid potential sex differences. The numbers of subjects in the subgroups were as follows: muscle CSA: Q1 = 441, Q2 = 438, Q3 = 444, and Q4 = 442; fat CSA: Q1 = 438, Q2 = 443, Q3 = 443, and Q4 = 441. Statistical significance was assessed by analysis of variance
A similar positive association between muscle mass and the ABI was observed in the Nagahama dataset regardless of the method of muscle mass measurement (Fig. 2). To clarify whether the clinical background of the elevated ABI subgroup differed according to the amount of SMI, we subdivided the participants by the 75th percentiles of the ABI (men ≥ 1.16; women ≥ 1.14) and SMI (men ≥ 8.14; women ≥ 6.56) because few individuals showed an ABI ≥ 1.4 (Table 1) and analyzed group differences in clinical parameters (Table 2). Compared with the control group, the elevated ABI with a normal SMI subgroup and the elevated ABI with a high SMI subgroup showed significantly different values for several parameters (Table 2). A multinomial logistic regression analysis was then performed to identify factors characterizing the elevated ABI subgroups (Table 3). The results indicated that the determinant with the greatest difference between the subgroups was the HOMA-IR, with coefficients that contrasted significantly according to the SMI. Similar results were also observed in a regression model including sex [ABI (Q4) – SMI (Q1–Q3): coefficient = 0.154, p = 0.004; ABI (Q4) – SMI (Q4): coefficient = –0.247, p = 0.003]. Figure 3 depicts the differences in the HOMA-IR among the subgroups. Individuals with both higher ABI and SMI values showed a significantly lower HOMA-IR in the adjusted analysis, suggesting that a clinical profile of an elevated ABI may not be deleterious when associated with a higher SMI. The SMI was inversely associated with the HOMA-IR (β = −0.236, p < 0.001) even after adjustments for age (β = −0.022, p = 0.027), sex (β = 0.155, p < 0.001), and BMI (β = 0.658, p < 0.001).
Association between the SMI and ABI in the Nagahama cohort. The skeletal muscle index (SMI, kg/m2) was calculated by dividing appendicular lean mass by body height squared. The quartiles of the SMI were calculated within sex and then combined. The number of study participants in each subgroup is shown in the columns. Statistical significance was assessed by analysis of variance
Differences in the HOMA-IR among subgroups according to the ABI and SMI. The values are the crude (a) and adjusted (b) means. The adjusted factors were age, body mass index, Brinkman index, systolic blood pressure, HDL cholesterol, triglycerides, and baPWV. Statistical significance was assessed by analysis of variance (a) or a linear regression model (b). The number of study participants in each subgroup is shown in the columns
Discussion
In this cross-sectional analysis of two different general populations, we verified that appendicular muscle mass was a strong determinant of the ABI. The clinical implication of an elevated ABI, particularly in the context of insulin resistance, may differ based on the amount of muscle mass.
In our previous study [11], we reported that thigh muscle mass was a significant positive determinant of the ABI in part of the AASC population (n = 407). The reproducibility of the previous findings in this extended analysis and in the independent population sample strongly supports the involvement of muscle mass in measurement of the ABI. Common characteristics of individuals with a high ABI in various studies, namely, larger body size and a higher frequency of men, may reflect potentially greater appendicular muscle mass.
Thigh fat CSA was inversely associated with the ABI. Furthermore, a regression analysis including both fat CSA and muscle CSA identified only muscle CSA as a determinant of the ABI. These results strongly suggested that the composition but not the size of the lower extremity is a determinant of the ABI. Although we found a strong difference in muscle mass based on the sex of the participants, we did not perform a sex-specific analysis because a sex-specific analysis could obscure a true relationship between muscle mass and the ABI by dichotomizing the correlation according to sex. However, as the association remained significant in a regression analysis adjusted for sex, the involvement of muscle mass in the ABI may not be a false-positive finding caused by combining the results of men and women in the present study.
In a subgroup analysis of the Nagahama population, the HOMA-IR was identified as a positive determinant of an elevated ABI in subjects with a normal SMI. The positive association of insulin resistance with the ABI was consistent with previous findings in epidemiological studies that reported a significantly higher frequency of type 2 diabetes in high ABI sub-populations [4,5,6,7, 14, 32]. Furthermore, the incidence of a high ABI in a general population in a 4-year follow-up period has also been reported to be associated with type 2 diabetes [33]. A high ABI is generally believed to be due to arterial calcification [2]. Arterial calcification reflects two distinct vascular changes [34], namely, intimal calcification, which is associated with atherosclerosis caused mainly by lipid accumulation, inflammation, and fibrosis, and medial calcification due to structural changes in the arterial wall. Because insulin resistance is an established risk factor for atherosclerosis, with possible mechanisms of decreased insulin-mediated vasodilation and increased smooth muscle cell proliferation and enzymatic glycosylation of structural proteins such as collagen, these pathological changes may be factors in the positive relationship between the HOMA-IR and a high ABI.
In contrast, the HOMA-IR showed an inverse association with an elevated ABI in the high SMI subgroup, possibly due to insufficient compression of the tibial artery by the appendicular muscles rather than structural changes in large arteries. Another reason may be the inverse association between the SMI and the HOMA-IR. The clinical implication of an elevated ABI may consequently differ based on the amount of muscle mass. Because previous longitudinal studies on cardiovascular outcomes did not consider the amount of muscle mass, the results of these studies may underestimate the true prognostic significance of a high ABI in poor cardiovascular outcomes. Since the present study used a cross-sectional design, we could not investigate the possibility that appendicular muscle mass may modulate the prognostic significance of a high ABI. The small number of individuals with an ABI > 1.4 was also a limitation for clarifying this issue. Although ethnic differences in arterial properties may partly account for the small number of individuals with a high ABI in our population, differences in body size and composition may be more plausible explanations for the lower frequency since the body size of Asians is known to be smaller than that of Europeans [35]. Another Japan-based study also reported a small number of individuals with a high ABI (n = 2) in a population of approximately 3000 [36].
An important limitation of this study warranting discussion was measurement of the ABI using an oscillometric method rather than a standard method using continuous wave Doppler ultrasound [37]. Although the reliability of oscillometric determination of the ABI has been confirmed [37, 38], a poor correlation with intra-arterial BP was also reported in obese individuals even when an appropriate cuff size was used [39]. In addition, we did not directly measure appendicular muscle mass in the Nagahama cohort. However, lean mass estimation using the SMF-BIA method has been found to be suitable for evaluating ALM [23, 24, 26,27,28].
In summary, we confirmed that appendicular muscle mass is a strong determinant of the ABI and may modulate the clinical significance of an elevated ABI. Careful attention is warranted in epidemiological studies of the ABI, particularly in those with obese individuals as well as muscular individuals and subjects with large body sizes.
References
Saji N, Kimura K, Yagita Y, Kawarai T, Shimizu H, Kita Y. Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res. 2015;38:323–8.
Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909.
Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208.
Ix JH, Katz R, Peralta CA, de Boer IH, Allison MA, Bluemke DA, Siscovick DS, Lima JA, Criqui MH. A high ankle brachial index is associated with greater left ventricular mass MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;55:342–9.
McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41.
Hendriks EJ, Westerink J, de Jong PA, de Borst GJ, Nathoe HM, Mali WP, van der Graaf Y, van der Schouw YT, Beulens JW, SMART Study Group. Association of high ankle brachial index with incident cardiovascular disease and mortality in a high-risk population. Arterioscler Thromb Vasc Biol. 2016;36:412–7.
Sutton-Tyrrell K, Venkitachalam L, Kanaya AM, Boudreau R, Harris T, Thompson T, Mackey RH, Visser M, Vaidean GD, Newman AB. Relationship of ankle blood pressures to cardiovascular events in older adults. Stroke. 2008;39:863–9.
Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1506–12.
Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9.
O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–93.
Tabara Y, Igase M, Kido T, Ochi N, Miki T, Kohara K. Composition of lower extremity in relation to a high ankle-brachial index. J Hypertens. 2009;27:167–73.
Sutliff RL, Walp ER, El-Ali AM, Elkhatib S, Lomashvili KA, O’Neill WC. Effect of medial calcification on vascular function in uremia. Am J Physiol Ren Physiol. 2011;301:F78–83.
Allison MA, Laughlin GA, Barrett-Connor E, Langer R. Association between the ankle-brachial index and future coronary calcium (the Rancho Bernardo study). Am J Cardiol. 2006;97:181–6.
Lilly SM, Jacobs DR Jr, Kronmal R, Bluemke DA, Criqui M, Lima J, Allison M, Duprez D, Segers P, Chirinos JA. Arterial compliance across the spectrum of ankle-brachial index: the Multiethnic Study of Atherosclerosis. Atherosclerosis. 2014;233:691–6.
Chen SC, Lee WH, Hsu PC, Huang JC, Lee CS, Lin TH, Voon WC, Lai WT, Sheu SH, Su HM. Association of body mass index and left ventricular mass index with abnormally low and high ankle-brachial indices in chronic kidney disease. Hypertens Res. 2016;39:166–70.
Tabara Y, Matsumoto T, Murase K, Nagashima S, Hirai T, Kosugi S, Nakayama T, Wakamura T, Chin K, Matsuda F; the Nagahama Study Group. Seasonal variation in nocturnal home blood pressure fall: the Nagahama study. Hypertens Res. 2018. doi: 10.1038/s41440-017-0003-3.
Tabara Y, Igase M, Miki T, Ohyagi Y, Matsuda F, Kohara K, J-SHIPP Study Group. B-type natriuretic peptide is a determinant of the nocturnal increase in blood pressure independently of arterial hypertrophy and hypoxia. J Hypertens. 2016;34:2393–401.
Tabara Y, Igase M, Miki T, Ohyagi Y, Matsuda F, Kohara K, J-SHIPP Study Group. Orthostatic hypertension as a predisposing factor for masked hypertension: the J-SHIPP study. Hypertens Res. 2016;39:664–9.
Tabara Y, Igase M, Okada Y, Nagai T, Miki T, Ohyagi Y, Matsuda F, Kohara K. Usefulness of the second derivative of the finger photoplethysmogram for assessment of end-organ damage: the J-SHIPP study. Hypertens Res. 2016;39:552–6.
Tabara Y, Takahashi Y, Kumagai K, Setoh K, Kawaguchi T, Takahashi M, Muraoka Y, Tsujikawa A, Gotoh N, Terao C, Yamada R, Kosugi S, Sekine A, Yoshimura N, Nakayama T, Matsuda F, Nagahama Study Group. Descriptive epidemiology of spot urine sodium-to-potassium ratio clarified close relationship with blood pressure level: the Nagahama study. J Hypertens. 2015;33:2407–13.
Kumagai K, Tabara Y, Yamashiro K, Miyake M, Akagi-Kurashige Y, Oishi M, Yoshikawa M, Kimura Y, Tsujikawa A, Takahashi Y, Setoh K, Kawaguchi T, Terao C, Yamada R, Kosugi S, Sekine A, Nakayama T, Matsuda F, Yoshimura N, Nagahama Study Group. Central blood pressure relates more strongly to retinal arteriolar narrowing than brachial blood pressure: the Nagahama study. J Hypertens. 2015;33:323–9.
Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16.
Demura S, Sato S, Kitabayashi T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J Physiol Anthropol Appl Human Sci. 2004;23:93–99.
Sillanpää E, Cheng S, Häkkinen K, Finni T, Walker S, Pesola A, Ahtiainen J, Stenroth L, Selänne H, Sipilä S. Body composition in 18- to 88-year-old adults—comparison of multifrequency bioimpedance and dual-energy X-ray absorptiometry. Obesity. 2014;22:101–9.
Seino S, Shinkai S, Iijima K, Obuchi S, Fujiwara Y, Yoshida H, Kawai H, Nishi M, Murayama H, Taniguchi Y, Amano H, Takahashi R. Reference values and age differences in body composition of community-dwelling older Japanese men and women: a pooled analysis of four cohort studies. PLoS ONE. 2015;10:e0131975.
Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, Maier AB. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–5.
Shafer KJ, Siders WA, Johnson LK, Lukaski HC. Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass indexes. Nutrition. 2009;25:25–32.
Völgyi E, Tylavsky FA, Lyytikäinen A, Suominen H, Alén M, Cheng S. Assessing body composition with DXA and bioimpedance: effects of obesity, physical activity, and age. Obesity . 2008;16:700–5.
Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–64.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–7.
Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–6.
Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–203.
Tison GH, Ndumele CE, Gerstenblith G, Allison MA, Polak JF, Szklo M. Usefulness of baseline obesity to predict development of a high ankle brachial index (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2011;107:1386–91.
Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605.
Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–90.
Kojima I, Ninomiya T, Hata J, Fukuhara M, Hirakawa Y, Mukai N, Yoshida D, Kitazono T, Kiyohara Y. A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb. 2014;21:966–73.
Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension. 2006;47:35–8.
Davies JH, Williams EM. Automated plethysmographic measurement of the ankle-brachial index: a comparison with the Doppler ultrasound method. Hypertens Res. 2016;39:100–6.
Umana E, Ahmed W, Fraley MA, Alpert MA. Comparison of oscillometric and intraarterial systolic and diastolic blood pressures in lean, overweight, and obese patients. Angiology. 2006;57:41–5.
Acknowledgements
The present study was supported by a university grant, the Center of Innovation Program, the Global University Project, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science & Technology in Japan; the Practical Research Project for Rare/Intractable Diseases, the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D from the Japan Agency for Medical Research and Development (AMED); a research grant from the Takeda Science Foundation; a research grant from the Japan Arteriosclerosis Prevention Fund; and a Research Promotion Award from Ehime University. We are extremely grateful to the Nagahama City Office and the non-profit organization Zeroji Club for their help in performing the Nagahama study. We also thank the editors of Crimson Interactive Pvt. Ltd for their help in the preparation of this manuscript.
Funding:
The present study was supported by a university grant, the Center of Innovation Program, the Global University Project, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science & Technology in Japan; the Practical Research Project for Rare/Intractable Diseases, the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D from the Japan Agency for Medical Research and Development (AMED); a research grant from the Takeda Science Foundation; a research grant from the Japan Arteriosclerosis Prevention Fund; and a Research Promotion Award from Ehime University.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
:The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tabara, Y., Igase, M., Setoh, K. et al. Clinical significance of an elevated ankle-brachial index differs depending on the amount of appendicular muscle mass: the J-SHIPP and Nagahama studies. Hypertens Res 41, 354–362 (2018). https://doi.org/10.1038/s41440-018-0020-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0020-x