Abstract
Striated muscle preferentially expressed protein kinase (SPEG) variants have been reported to cause centronuclear myopathy associated with cardiac diseases. The severity of skeletal muscle symptoms and cardiac symptoms are presumably related to the location of the variant. Here, we report novel SPEG compound heterozygous pathological variants in a neonate with severe dilated cardiomyopathy and relatively mild hypotonia. This report expands the genotype-phenotype correlations of patients with SPEG variants.
Similar content being viewed by others
Centronuclear myopathy (CNM) is a form of congenital myopathy characterized by muscle weakness and centralized skeletal muscle nuclei on biopsy1. The clinical presentation of patients ranges from severe muscle weakness requiring respiratory support to mild hypotonia. Recessive variants in striated muscle preferentially expressed protein kinase (SPEG), a member of the myosin light chain kinase family, have recently been reported to cause a CNM phenotype with or without cardiac complications, such as dilated cardiomyopathy (DCM)2,3,4. It is known that some patients have more severe cardiac symptoms than skeletal muscle symptoms, but what determines their phenotype remains unclear. We herein report novel SPEG compound heterozygous pathological variants in a neonate with severe DCM and relatively mild hypotonia. This report expands the genotype-phenotype correlations of patients with SPEG variants.
The patient was a boy born at 36 weeks and 6 days of gestation with a birth weight of 2668 g (45.1 percentile) who was the second child of nonconsanguineous parents. There was no family history of neuromuscular disease (Fig. 1A). During pregnancy, antenatal ultrasound examination revealed pleural effusion and ascites, and he was suspected to have left ventricular noncompaction. After he was born by spontaneous vaginal delivery, he was admitted to the neonatal intensive care unit for close examination. On admission, he showed severe hypotonia (Fig. 1B), and echocardiography detected abnormal trabeculation of the left ventricle with marked thickening of the noncompacted layer, which was consistent with left ventricular noncompaction. The amount of pericardial effusion was small, and his cardiac function was preserved. The amount of pleural effusion increased gradually, and drainage revealed chylothorax. After administration of medium-chain fatty acid milk, the effusion decreased. The patient was temporarily on tube feeding due to hypotonia, but his muscle tone improved by approximately two weeks after birth, and he was gradually able to feed orally. However, his cardiac function deteriorated three weeks after birth, and dilation of the left ventricle became apparent (Fig. 1C, D). His cardiac function was not improved by intravenous inotropic agents or diuretics, and we switched to chronic heart failure management with beta blockers, angiotensin II receptor blockers and phosphodiesterase inhibitors. Myocardial biopsy performed during cardiac catheterization revealed fibrosis of the interstitium associated with dropout of cardiomyocytes (Fig. 1E). Despite careful adjustment of medications, he died at five months of age due to heart failure.
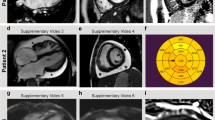
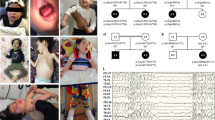
A The family pedigree. The arrow shows the patient reported. B A photograph of the patient taken at one week of age. At this time, he was floppy and needed tube feeding. X-ray (C) and echocardiography (D) of the patient when heart failure was detected showing cardiac enlargement, ventricular dilation and decreased cardiac contractility. E Elastica‐Picrosirius red staining of the myocardium. The red stained area shows fibrosis of the interstitium associated with myocardial dropout. F Sanger sequencing showing the variants detected, revealing that c.5343 G > A (p.(Trp1781*)) was inherited from the mother and that c.9391 G > A (p.(Glu3131Lys)) was inherited from the father. The variant marked with an arrowhead next to c.9391 G > A (arrow) is a single-nucleotide polymorphism derived from his mother that has no pathological significance. G A schema of the full-length striated muscle preferentially expressed protein kinase (SPEG) protein showing the protein kinase domain and MTM1-interacting region. The variants reported thus far are indicated (homozygous variants in bold). Variants causing severe symptoms of both the heart and skeletal muscle (P1-2) are shown in red boxes with solid lines. Those with severe cardiac symptoms and milder or no skeletal muscle symptoms (P3-8 and our case) are shown in pink boxes with dotted lines. Those with skeletal muscle symptoms but with milder or no cardiac symptoms (P9-12) are shown in green boxes with dashed lines.
A chromosome test was performed to search for underlying disease, which revealed a normal male karyotype (46,XY). Newborn screening for metabolic disorders was negative. Next, we performed whole-exome sequencing. After obtaining written informed consent from his parents, we collected blood samples from the patient. Genomic DNA was extracted from peripheral blood samples following a standard protocol, and exome sequencing was performed for the patient using an Ion AmpliSeq Exome RDY kit (Thermo Fisher Scientific) according to the manufacturer’s protocols. The results showed a nonsense variant [NM_005876.5: c.5343 G > A: p.(Trp1781*)] and a missense variant [NM_005876.5: c.9391 G > A: p.(Glu3131Lys)] in SPEG, neither of which have been previously reported in Genome Aggregation Database (gnomAD, URL: http://gnomad.broadinstitute.org/). Standard Sanger sequencing was performed for the patient and parents, revealing that p.(Trp1781*) and p.(Glu3131Lys) were inherited from the mother and the father, respectively (Fig. 1F). The patient’s phenotype matched the known spectrum of clinical features for SPEG variants. The maternally derived nonsense variant is predicted to be pathogenic according to American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines (PVS1: nonsense, PM2: absent from controls, PP4: patient’s phenotype)5. The paternally derived missense variant is predicted to be damaging by in silico evaluation tools, Combined Annotation Dependent Depletion (CADD score 31) and PolyPhen-2 (score 1.000) and is interpreted as likely pathogenic according to guidelines (PM2: absent from controls, PM3: in trans with a pathogenic variant, PP3: in silico evaluation, PP4: patient’s phenotype)5.
SPEG plays a significant role in muscle development and regeneration. In addition, it regulates cardiac and skeletal muscle calcium homeostasis and is involved in both acquired and genetic cardiovascular disease3. SPEG has two protein kinase domains, 1 and 2, which are reported to be key domains that control Ca2+ reuptake6,7. To date, 15 pathogenic variants of SPEG have been reported3,4,8,9,10,11,12,13. The 12 variants that were evaluated and monitored for both neuromuscular manifestations and cardiac abnormalities are shown in Fig. 1G and Table 1, along with data from our patient. We defined “severe myopathy” as a group of patients with severe muscle weakness requiring respiratory support and “mild or no myopathy” as a group of patients with a milder phenotype. Because we did not conduct skeletal muscle biopsy or postmortem pathology autopsy, we presumed that the patient had CNM due to the presence of hypotonia.
It has been hypothesized that the phenotype of patients with SPEG variants may be predicted by the location of the variants. This is explained by two known theories. First, the severity of cardiac comorbidities has been suggested to be linked to variants affecting both isoforms expressed in skeletal and cardiac muscle4,12. Second, a variant that involves a certain region in SPEG called the MTM1-interacting region is assumed to contribute to the severity of skeletal muscle symptoms2,8.
Two of the four isoforms of SPEG, SPEGα and SPEGβ, are expressed in skeletal and cardiac muscle14 and play a critical role in regulating their contraction by phosphorylating Ca2+-handling proteins and maintaining the transverse tubule3. The severity of cardiac comorbidities is suggested to be linked to variants affecting both SPEGα and β4,12. If the variant in at least one allele is in a position involving only SPEGβ, cardiac function tends to be mostly preserved because it is complemented by SPEGα (Fig. 1G, Table 1). Consistent with this theory, we observed that Patients (P) 9-12 had preserved cardiac function but that P1-8 exhibited severe cardiac dysfunction, such as DCM. In our case, the severe cardiac manifestation might be explained by the effects of the variants on both the α and β isoforms.
On the other hand, some patients have milder skeletal dysfunction compared to their severe cardiac dysfunction. This is presumably caused by the MTM1-interacting region of SPEG2,8. SPEG variants were first reported to cause the CNM phenotype in the process of investigating MTM1 function and related proteins2. In striated muscle, the protein encoded by MTM1, a causative gene of X-linked myotubular myopathy, interacts and colocalizes with a particular region in SPEG (NM_005876.5:c.2530-2674). This MTM1-interacting region in SPEG plays a critical role in excitation-contraction coupling and cytoskeletal organization2. For patients who have variants affecting both SPEGα and β isoforms, it has been speculated that patients, such as P1-P2 with truncating mutations N-terminal to the MTM1-interacting region tend to have severe myopathic symptoms, such as the need for respiratory support. In contrast, patients, such as P3-P8, who have one or both alleles with an intact MTM1-interacting region present with relatively mild or no skeletal muscle symptoms, suggesting that missense variants or deletions are unlikely to cause a change in the 3D structure (Fig. 1G, Table 1)2,8,13. In our case, the MTM1-interacting region of the allele of the missense variant p.(Glu3131Lys) should be intact because the variant is located outside of the MTM1-interacting region. The other variant in our case, p.(Trp1781*), is located N-terminal to the MTM1-interacting region, and a truncated protein should not contain the MTM1-interacting region and might be affected by nonsense-mediated mRNA decay. Relatively mild myopathic symptoms in a patient may be explained by the presence of the MTM1-interacting region.
In conclusion, novel compound heterozygous SPEG variants were identified in an infant developing dilated cardiomyopathy who had transient hypotonia. The variants and phenotype in this case support and reinforce the hypothesis that has been postulated in previous reports and expand the genotype-phenotype correlations of patients with SPEG variants.
HGV database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.3324. https://doi.org/10.6084/m9.figshare.hgv.3327.
References
Jungbluth, H., Wallgren-Pettersson, C. & Laporte, J. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 3, 26 (2008).
Agrawal, P. et al. SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am. J. Hum. Genet 95, 218–226 (2014).
Campbell, H., Aguilar-Sanchez, Y., Quick, A., Dobrev, D. & Wehrens, X. SPEG: a key regulator of cardiac calcium homeostasis. Cardiovasc Res 117, 2175–2185 (2021).
Luo, S., Rosen, S., Li, Q. & Agrawal, P. Striated preferentially expressed protein kinase (SPEG) in muscle development, function, and disease. Int J. Mol. Sci. 22, 5732 (2021).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17, 405–424 (2015).
Quick, A. et al. ‘Striated muscle preferentially expressed protein kinase’ (SPEG) is essential for cardiac function by regulating junctional membrane complex activity. Circ. Res 120, 110–119 (2017).
Quan, C. et al. SPEG controls calcium reuptake into the sarcoplasmic reticulum through regulating SERCA2a by its second kinase-domain. Circ. Res 124, 712–726 (2019).
Gurgel-Giannetti, J. et al. A novel SPEG mutation causing congenital myopathy with fiber size disproportion and dilated cardiomyopathy with heart transplantation. Neuromuscul. Disord. 31, 1199–1206 (2021).
Levitas, A. et al. A novel mutation in SPEG causes early onset dilated cardiomyopathy. PLoS Genet 16, e1009000 (2020).
Zhang, G. et al. Clinical and genetic analysis of a case with centronuclear myopathy caused by SPEG gene mutation: a case report and literature review. BMC Pediatr. 2181, 209 (2021).
Jaouadi, H. et al. Dilated-left ventricular non-compaction cardiomyopathy in a pediatric case with SPEG compound heterozygous variants. Int J. Mol. Sci. 23, 5205 (2022).
Qualls, A. et al. Novel SPEG mutations in congenital myopathies: genotype-phenotype correlations. Muscle Nerve 59, 357–362 (2019).
Wang, H. et al. Insights from genotype-phenotype correlations by novel SPEG mutations causing centronuclear myopathy. Neuromuscul. Disord. 27, 836–842 (2017).
Hsieh, C. et al. Striated muscle preferentially expressed genes alpha and beta are two serine/threonine protein kinases derived from the same gene as the aortic preferentially expressed gene-1. J. Biol. Chem. 275, 36966–36973 (2000).
Acknowledgements
We are grateful to the patient and his family for providing samples and clinical histories. This study was partly supported by the AMED grant JP21gk0110038 for SS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujimoto, H.M., Fujimoto, M., Sugiura, T. et al. Novel SPEG variants in a neonate with severe dilated cardiomyopathy and relatively mild hypotonia. Hum Genome Var 10, 24 (2023). https://doi.org/10.1038/s41439-023-00253-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-023-00253-w