Abstract
Anthocyanins play vital roles in plant stress tolerance and growth regulation. Previously, we reported that the photomorphogenesis-related transcription factor SlBBX20 regulates anthocyanin accumulation in tomato. However, the underlying mechanism remains unclear. Here, we showed that SlBBX20 promotes anthocyanin biosynthesis by binding the promoter of the anthocyanin biosynthesis gene SlDFR, suggesting that SlBBX20 directly activates anthocyanin biosynthesis genes. Furthermore, we found by yeast two-hybrid screening that SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2, and the interaction was confirmed by bimolecular fluorescence complementation and coimmunoprecipitation assays. SlCSN5 gene silencing led to anthocyanin hyperaccumulation in the transgenic tomato calli and shoots, and SlCSN5-2 overexpression decreased anthocyanin accumulation, suggesting thSlCSN5-2 enhanced the ubiquitination of SlBBX20 and promoted the degradation of SlBBX20 in vivo. Consistently, silencing the SlCSN5-2 homolog in tobacco significantly increased the accumulation of the SlBBX20 protein. Since SlBBX20 is a vital regulator of photomorphogenesis, the SlBBX20-SlCSN5-2 module may represent a novel regulatory pathway in light-induced anthocyanin biosynthesis.
Similar content being viewed by others
Introduction
Anthocyanins are pigments synthesized by the flavonoid pathway involved in the coloring of various organs, such as leaves, fruits, and flowers1,2. Anthocyanins accumulate in response to plant hormones, low temperature, high temperature, strong light, UV-B radiation, and other environmental factors2,3,4,5,6,7,8. Moreover, fruit color is a significant indicator of quality. Anthocyanins are the main pigments responsible for determining color in a broad variety of fruits. Anthocyanins also play significant roles as antioxidants in plant biotic and abiotic stress tolerance and thereby facilitate plant resistance to pathogens and insects.
Aft and Atv are two important loci that regulate anthocyanin biosynthesis in tomato. The Aft gene from tomato was mapped to chromosome 10 and found to encode a SlAN2-like R2R3-MYB protein that promotes anthocyanin biosynthesis9,10. Atv is located on chromosome 7 and encodes the SlMYBATV protein, which negatively regulates the synthesis of anthocyanins. In addition to positively regulating anthocyanin biosynthesis, Aft was reported to directly activate SlMYBATV expression. Additionally, SlMYBATV competes with Aft for interaction with the transcription factor SlJAF13, thereby downregulating the accumulation of anthocyanins in tomato fruit. Mutation of SlMYBATV results in the release of SlJAF13, which interacts with Aft, further leading to the upregulation of SlAN1 and SlAN11 expression and accumulation of anthocyanins in tomato fruit9,11.
Anthocyanin accumulation is mostly regulated by transcription factors and structural genes, including CHS, CHI, F3H, F3’H, F3’5’H, DFR, ANS, and UFGT8,12. The biosynthesis of anthocyanins is regulated by different transcription factors. The MYB-bHLH-WD40 (MBW) complex plays vital roles in regulating the biosynthesis of anthocyanins. The molecular mechanism by which the MBW complex regulates anthocyanin biosynthesis has been extensively studied. The WD40 protein likely plays a more general role in the regulation of the complex8,13. The activation of particular genes is determined by the expression pattern and DNA-binding specificity of the MYB and bHLH proteins.
SlAN2 was reported to promote anthocyanin biosynthesis when plants were grown in strong light and under low-temperature conditions14. A recent study showed that the overexpression of SlMYB75 induced the accumulation of anthocyanins15. Overexpression of SlANT1 was reported to increase the expression levels of structural genes in the anthocyanin biosynthesis pathway16. The MdMYB1/10 gene was found to be involved in regulating the accumulation of anthocyanins in apple17,18. The MYB-TF gene Cs6g17570 was identified as a vital player in the regulation of anthocyanin biosynthesis in blood oranges19. MdMYB16 and MdbHLH33 were also reported to be involved in anthocyanin metabolism20. In addition, MdbHLH3 was found to promote anthocyanin accumulation and fruit coloring under low-temperature conditions in apple21. The WD40 protein MdTTG1 was reported to interact with bHLH to regulate anthocyanin synthesis in apple22.
B-box (BBX) proteins are a class of zinc finger protein transcription factors that contain one or two B-box domains. Many studies in Arabidopsis have revealed that BBX family proteins play an important role in photomorphogenesis23. This particular group of BBX factors includes AtBBX1, AtBBX4, AtBBX19, AtBBX20, AtBBX21, AtBBX22, AtBBX23, AtBBX24, AtBBX25, AtBBX28, AtBBX30, AtBBX31 and AtBBX3224,25,26,27,28,29,30,31. The accumulation of anthocyanin is a general phenomenon in photomorphogenesis, and BBX proteins were also found to regulate anthocyanin synthesis. In pear, the BBX proteins PpBBX16, PpBBX18, PpBBX21, and PpBBX24 are involved in anthocyanin accumulation5,6,32. In apple, MdCOL4, MdBBX20, MdBBX22, and MdBBX37 were found to participate in the regulation of anthocyanin accumulation2,3,4,33.
In a previous study, we found that the SlBBX20 protein is modified by the CRL4 E3 ubiquitin ligase to regulate the biosynthesis of carotenoids in tomato fruit34. In addition to the carotenoid content, we found that overexpression of the SlBBX20 gene led to a significant increase in the anthocyanin content. Here, SlBBX20 was found to target the DFR promoter and activate its expression. To further uncover the mechanism by which anthocyanin biosynthesis is regulated, we screened a yeast two-hybrid library and found that SlCSN5-2 interacts with SlBBX20. The downregulation of SlCSN5-2 resulted in the accumulation of anthocyanin in tomato. Furthermore, when we interfered with the expression of a SlCSN5-2 homolog in tobacco, the expression level of the SlBBX20 protein was significantly increased, indicating that SlCSN5-2 regulates the accumulation of the SlBBX20 protein.
Results
Overexpression of SlBBX20 led to increased anthocyanin accumulation
To study the role of SlBBX20 in regulating the accumulation of anthocyanins, we overexpressed full-length SlBBX20 in tomato with the 35S promoter. In the transformation process, we found that the partial calli growing on the medium had become purple (Fig. 1a). After rooting, the seedlings and roots of the SlBBX20-overexpressing plants were also purple (Fig. 1b, c). Purple sepals in the flowers of SlBBX20-overexpressing plants were also observed (Fig. 1d). Thus, anthocyanins accumulated in diverse tissues with the overexpression of SlBBX20.
We also obtained three homozygous SlBBX20-knockout plants using CRISPR-Cas9 technology. No obvious difference in anthocyanin content was observed between the knockout plants and control plants, which may be due to redundant gene functions. Recent studies have shown that Arabidopsis BBX20, 21 and 22 are functionally redundant and regulate hypocotyl elongation and anthocyanin accumulation as rate-limiting cofactors of HY535. Therefore, we selected SlBBX20-overexpressing plants to perform follow-up experiments.
We quantified the expression of SlBBX20 and measured the anthocyanin content in 14 independent transgenic lines (Fig. 2a–c). The level of SlBBX20 transcription was highly correlated with anthocyanin content. We further used the Pearson correlation coefficient to assess the correlation between these two parameters (Fig. 2d). The scatter plot showed the Pearson correlation coefficient between the relative SlBBX20 expression level and level of accumulated anthocyanin to be 0.84, indicating a strong positive correlation between them.
a Relative SlBBX20 expression levels in different SlBBX20-overexpressing lines. b Extracts containing anthocyanins derived from the leaves of the corresponding SlBBX20-overexpressing lines. c Anthocyanin content in the leaves of the corresponding SlBBX20-overexpressing lines. d Scatter plot showing the correlation between relative SlBBX20 expression and anthocyanin content. The bars show the mean ± SE (n = 3). A One-way ANOVA and Dunnett’s test were conducted. “*” and “**” indicate statistically significant differences with P < 0.05 and P < 0.01, respectively
The expression of flavonoid biosynthesis genes was upregulated in SlBBX20-overexpressing lines
To explore the molecular mechanism by which SlBBX20 regulates anthocyanin accumulation, we analyzed the transcriptomes of the SlBBX20-overexpressing plants and controls by RNA sequencing (RNA-Seq). Anthocyanins are synthesized by the flavonoid pathway. The expression levels of some genes in the pigment, flavonoid and anthocyanin biosynthesis pathways (DFR, ANS, CHS1, CHS2, F3H, F3'5’H, and FLS) were found to be upregulated in the SlBBX20-overexpressing plants (Fig. 3a). To independently validate these results, we quantified the expression of these anthocyanin-related structural genes in two SlBBX20-overexpressing lines, OE-20 and OE-40 (Fig. 3b). The expression levels of CHS1, CHS2, F3H, F3'5’H, DFR, and FLS were found to be upregulated in the SlBBX20-overexpressing lines relative to the control line. Among these genes, DFR, ANS, and F3'5’H were elevated more than twofold. This result is consistent with the transcriptome data, and these data imply that SlBBX20 promotes the accumulation of anthocyanins by regulating the expression of anthocyanin biosynthesis genes.
a Heatmap of pigment-associated genes with increased expression in SlBBX20-overexpressing lines identified from an analysis of transcriptome data. b Validation of the transcriptome data using qRT-PCR. We validated the expression levels of structural genes associated with anthocyanin biosynthesis that were classified as upregulated based on an analysis of our transcriptome data
SlBBX20 directly regulates SlDFR
We hypothesized that SlBBX20 directly regulates transcription of the anthocyanin biosynthesis genes that were upregulated in the SlBBX20-overexpressing lines. BBX proteins were reported to regulate their target genes by binding G-boxes in their promoters. The cis-elements in the promoters of DFR, ANS, CHS1, CHS2, F3H, F3'5’H, and FLS were analyzed by using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)36. The promoter of ANS does not contain a G-box. Therefore, we speculated that SlBBX20 cannot bind the ANS promoter. Yeast one-hybrid assays were conducted to test whether SlBBX20 regulates the other genes in this group. The yeast strain Y1GOLD was cotransformed with AD-SlBBX20 and pAbAi-CHS1, pAbAi-CHS2, pAbAi-F3H, pAbAi-F3'5’H, pAbAi-FLS or a negative control. None of these transformants survived on the selective medium, which lacked Leu and Ura and contained AbA. These data indicate that SlBBX20 could not interact with the promoters of CHS1, CHS2, F3H, F3'5’H, or FLS, although these promoters contain G-boxes (data not shown). The promoter of SlDFR contains three G-boxes (Fig. 4a). We designed the G-box1, 2 and 3 sequences to confirm their interaction. We found that Y1GOLD yeast cells cotransformed with AD-SlBBX20 and pAbAi-SlDFR (including G-box1, 2) could survive on the selective medium. However, pAbAi-SlDFR including G-box3 could not survive on the medium (data not shown). These data indicate that SlBBX20 can bind G-box1 or 2 in the promoter of SlDFR (Fig. 4b).
a Schematic diagram of the G-box locations on the DFR promoter. P1 includes G-box1 and G-box2, and the black dots indicate the locations of the G-boxes. b SlBBX20 was shown to bind the DFR promoter in a yeast one-hybrid assay. The yeast strain Y1HGold was transformed with the bait vector pAbAi-DFR and the prey vector AD-SlBBX20 and plated on SD -Leu-Ura medium with or without aureobasidin A (45 ng mL−1). c Wild-type and mutant probe sequences used for EMSAs. G-box1-wt and G-box2-wt were used as wild-type probes. The synthesized G-box1-mt and G-box2-mt sequences were mutation probes in which the cis-element sequences were replaced by GGGGGG or GGGGGGG, respectively. d Affinity of SlBBX20 for two G-box motifs. BP indicates the binding probe; FP indicates the free probe. “+“ and “-“ indicate presence or absence in the EMSA, respectively. e Schematic of the vector used for the dual-luciferase experiment. f LUC/REN ratios. Luciferase activity was detected by dual-luciferase reporter assay
Next, we performed an EMSA to determine which G-box (G-box1 or 2) is targeted by SlBBX20. In the EMSAs, the SlBBX20 protein bound the G-box1-wt probe but not the other probes. This result demonstrated that SlBBX20 binds G-box1 in the SlDFR promoter in vitro (Fig. 4c, d). Subsequently, a dual-luciferase system assay was performed to test whether SlBBX20 could activate the expression of SlDFR. As shown in Fig. 4f, the ratio of LUC to REN in tobacco leaves co-injected with 62SK-SlBBX20 and pAbAi-SlDFR was increased by 2.6-fold relative to the negative control (62SK and pAbAi-SlDFR). These results provide evidence that SlBBX20 can activate the transcription of SlDFR by binding the G-box1 cis-element in its promoter.
SlBBX20 interacts with SlCSN5-2 in vivo
To further analyze the molecular mechanism by which SlBBX20 regulates anthocyanin content, a yeast two-hybrid (Y2H) screen was performed using SlBBX20 as bait. We found that the protein encoded by Solyc06g073150 could interact with SlBBX20 in the Y2H screen. The full-length coding sequence of this gene is 1104 bp in length. The gene encodes a predicted protein consisting of 367 amino acid (aa) residues, which was referred to as SlCSN5-2 in a previous study37. We used three different methods to confirm the interaction between the SlBBX20 and SlCSN5-2 proteins. First, the interaction was verified using the Y2H assay (Fig. 5a). The full-length SlCSN5-2 protein was previously demonstrated to generate false-positive results in the Y2H assay. Therefore, a truncated SlCSN5-2 construct (SlCSN5-257-367) was used to test the interaction. SlBBX20 was also divided into two fragments consisting of residues 56-203 or 101-203. The yeast cells cotransformed with the BD-SlBBX20/BD-SlBBX2056-203 and AD-SlCSN5-257-367 plasmids grew on SD -Leu/Trp/His/Ade medium, but the negative control yeast cells and yeast cells cotransformed with BD-SlBBX20101-203 and AD-SlCSN5-257-367 did not grow on this medium, suggesting an interaction between SlBBX2056-101 and SlCSN5-257-367.
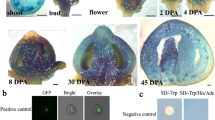
a Interaction between SlBBX20 and SlCSN5-2 in yeast two-hybrid experiments. The BD-S1BBX20 and AD-S1CSN5-257-367 (residues 57 to 367) plasmids were cotransformed into yeast strain AH109. b Interaction between SlBBX20 and SlCSN5-2 in coimmunoprecipitation assays. SlBBX20-HA and SlCSN5-2-FLAG were simultaneously introduced into tobacco protoplasts. The proteins were immunoprecipitated using anti-FLAG matrix beads and analyzed by western blotting. c Interaction between SlBBX20 and SlCSN5-2 in BiFC assays. The pHBT-SlBBX20-nYFP and pHBT-SlCSN5-2-cYFP plasmids were cotransformed into Arabidopsis protoplasts. The pHBT-SlBBX20-nYFP and cYFP plasmids or pHBT-SlCSN5-2-cYFP and nYFP plasmids as a negative control were used. YFP fluorescence was observed using confocal laser scanning microscopy. d Subcellular localization of SlCSN5-2. SlCSN5-2 without a stop codon was cloned into pHBT-GFP. Tobacco protoplasts were extracted and cotransformed with plasmids encoding SlCSN5-2-GFP and a nuclear marker fused to RFP
Second, we employed a coimmunoprecipitation assay to confirm the interaction (Fig. 5b). SlBBX20-HA and SlCSN5-2-FLAG plasmids were cotransformed into tobacco protoplasts and expressed for 8–10 h. The proteins were immunoprecipitated using an anti-FLAG antibody and immunoblotted using an anti-HA antibody. The SlBBX20-HA protein was coimmunoprecipitated with SlCSN5-2-FLAG, but the negative control was not (Fig. 5b).
Finally, the interaction between SlBBX20 and SlCSN5-2 was tested using the bimolecular fluorescence complementation (BiFC) assay. We observed YFP fluorescence in tobacco protoplast cells cotransformed with the SlBBX20-nYFP and SlCSN5-2-cYFP plasmids but not in the negative control tobacco protoplast cells cotransformed with the empty vector and SlBBX20-nYFP or SlCSN5-2-cYFP plasmids (Fig. 5c). These data provide in vivo evidence that SlBBX20 interacts with SlCSN5-2.
We also determined the subcellular localization of SlCSN5-2, and the results showed that SlCSN5-2 accumulates in both the nucleus and cytoplasm (Fig. 5d). This localization pattern was very similar to that of SlBBX2034, suggesting that SlCSN5-2 and SlBBX20 work together.
SlCSN5 regulates anthocyanin accumulation
To study the effects of SlCSN5-2 on anthocyanin biosynthesis, we used RNA interference to downregulate the expression of SlCSN5 (including SlCSN5-1 and SlCSN5-2) in stably transformed plants. It was impossible to independently silence SlCSN5-1 and SlCSN5-2 in tomato due to their high sequence similarity37. While generating the transgenic plants, we observed many purple calli and shoots (Fig. 6a), similar to the effects of SlBBX20 overexpression. Strongly SlCSN5-silenced calli accumulated many anthocyanins but failed to grow into normal plants. The moderately silenced plants survived, but their growth was significantly restrained (Fig. 6b, c), indicating that the function of SlCSN5-2 in growth and development is indispensable. We selected a line in which SlCSN5 expression was moderately decreased to test the anthocyanin content and found that it was significantly increased compared with that in the WT plants (Fig. 6d). Correspondingly, when SlCSN5-2 was overexpressed in tomato, the anthocyanin content was significantly decreased compared with that in the WT plants (Fig. 6e), suggesting that SlCSN5 is a negative regulator of anthocyanin biosynthesis.
Calli (a) and seedlings (b) after SlCSN5 interference. c Detection of SlCSN5 expression in the SlCSN5-RNAi seedlings. d Anthocyanin content in the SlCSN5-RNAi plants. e Anthocyanin content in the SlCSN5-2-overexpressing plants. f The relative expression level of SlDFR in the SlCSN5-RNAi seedlings. Data represent the mean ± SE (n = 3). One-way ANOVA was performed. “*” and “**” indicate statistically significant differences with P < 0.05 and P < 0.01, respectively
Furthermore, to confirm whether SlCSN5-2 regulates the accumulation of anthocyanin by DFR, we detected the expression level of SlDFR in SlCSN5-RNAi plants. The expression level of SlDFR was significantly upregulated compared with that of the WT plants, and the expression level of SlCSN5-2 was negatively correlated with that of SlDFR (Fig. 6f). This result suggests that SlCSN5 negatively regulates the accumulation of anthocyanin in tomato by SlDFR.
SlCSN5-2 regulates accumulation of the SlBBX20 protein
Previously, we revealed that SlBBX20 is modulated by the E3 ubiquitin ligase CRL434. SlCSN5-2 was reported to regulate CRL ubiquitin ligase and may regulate ubiquitination of the SlBBX20 protein by modifying the E3 ubiquitin ligase CRL4. To explore whether SlCSN5-2 affects ubiquitination of the SlBBX20 protein, we conducted an immunoprecipitation assay to detect the ubiquitination of SlBBX20 with an anti-UBQ antibody. The results showed that upon SlCSN5-2 coexpression, ubiquitination of the SlBBX20 protein was enhanced, and expression of the SlBBX20 protein decreased with actin used as an input control (Fig. 7a).
a The effect of SlCSN5-2 on ubiquitination of the SlBBX20 protein. The SlBBX20-HA and SlCSN5-2-FLAG plasmids were cotransformed into tobacco protoplasts. Anti-FLAG matrix beads were used for immunoprecipitation experiments. Finally, several antibodies (anti-HA, anti-FLAG, and anti-UBQ) were used to detect the accumulation of different proteins, and actin was used as a control. b Relative NbCSN5B expression level in NbCSN5B-VIGS plants. Tobacco leaves were infiltrated with pTRV2-NbCSN5B and pTRV2 as controls. c NbCSN5B downregulated the SlBBX20-HA protein. NbCSN5B was silenced in tobacco by virus-induced gene silencing. The protoplasts were extracted and assessed using anti-HA, TRV2 as a control
NbCSN5B in tobacco is an ortholog of SlCSN5-2, and their gene sequences are highly similar (Supplementary Fig. S1). We used VIGS to silence NbCSN5B in tobacco. Relative NbCSN5B gene expression was confirmed to be downregulated in leaves (Fig. 7b). Then, we extracted protoplasts from tobacco and transiently expressed SlBBX20-HA in the protoplasts. After 10 h, the proteins were extracted and detected by western blotting (Fig. 7c). We found that the SlBBX20-HA protein accumulated to higher levels in the NbCSN5B-silenced plants than in the control plants. In general, these results suggest that SlCSN5-2 interacts with SlBBX20 and promotes its ubiquitination and degradation to negatively regulate anthocyanin biosynthesis.
Discussion
Anthocyanins are natural plant pigments involved in regulating the coloration of specific plant organs, such as leaves, flowers, and fruits. Previous studies on the regulation of anthocyanin biosynthesis have mainly focused on the MBW complex. Some positive regulators of anthocyanin biosynthesis, such as SlAN2, SlAN1, and SlMYB7514,15, and some negative regulators of anthocyanin biosynthesis, such as MdMYB16, FaMYB1, and PhMYB2720,38,39, have been reported in different plants. Recently, some BBX proteins in apple (i.e., MdCOL4, MdBBX20, MdBBX22) were found to regulate anthocyanin biosynthesis2,3,4,33. In pear, PpBBX16, PpBBX18, PpBBX21, and PpBBX24 were reported to be involved in anthocyanin synthesis5,6,32. A recent study showed that MdBBX37 inhibits the transactivating activities of MdMYB1 and MdMYB9 and therefore downregulates anthocyanin biosynthesis3.
In this study, we found that SlBBX20 interacts with SlCSN5-2 and promotes anthocyanin biosynthesis by binding the DFR promoter. Tomato plants overexpressing SlBBX20 accumulated high levels of anthocyanins. Anthocyanins are synthesized by the flavonoid pathway, which contains genes that contribute to anthocyanin biosynthesis at both the early and late stages40. We found that most of these genes were upregulated in the SlBBX20-overexpressing lines, although the extent to which they were upregulated varied. BBX transcription factors were reported to regulate transcription by binding G-box cis-elements4,6,33. Here, SlBBX20 was found to directly bind the first G-box in the SlDFR promoter and activate its expression, and the accumulation of anthocyanins was highly correlated with the expression level of SlBBX20. DFR is a key enzyme in the anthocyanin synthesis pathway, its mutation blocks the accumulation of anthocyanin in tobacco, and a white flower phenotype appeared41. MdBBX20 was reported to promote anthocyanin biosynthesis by binding the promoters of DFR, ANS, and MYB12. PpBBX16 requires PpHY5 to increase the expression levels of genes related to anthocyanin biosynthesis5. Thus, the mechanisms used by BBX20 to promote anthocyanin biosynthesis appear to be similar but vary among different plant species.
A large number of studies in Arabidopsis have shown that BBX family proteins are involved in photomorphogenesis26,27,28,29,30,31. Our previous study showed that SlBBX20-overexpressing tomato plants exhibited enhanced photomorphogenesis34. We identified the SlCSN5-2 protein as a binding partner of SlBBX20. The COP9 signalosome (CSN) plays an important role in plant photomorphogenesis and was originally discovered by cloning mutant alleles that disrupt photomorphogenesis in Arabidopsis42. The accumulation of anthocyanins is an important phenomenon in photomorphogenesis. Recently, several BBX proteins were found to interact with the HY5 protein and regulate anthocyanin accumulation in Arabidopsis, apple, and pear3,6,35. UV-B radiation induces the accumulation of anthocyanins using a signaling mechanism that depends on MdCOP1; This mechanism activates MdHY5 and promotes the binding of MdHY5 to the MdMYB gene-promoter region7. In this study, we found that CSN5-2—a photomorphogenesis factor—could negatively regulate the accumulation of anthocyanins by promoting the accumulation of the SlBBX20 protein. Although the SlBBX20-OE plants accumulated many anthocyanins under light, anthocyanin accumulation in the SlBBX20-OE plants was reduced upon exposure to dark conditions for a period of time, and the expression of SlDFR was significantly downregulated compared with that under light (Supplementary Fig. S2a, b, d). suggested that the anthocyanin modulation by SlBBX20 is dependent on light. The transcription level of SlCSN5-2 was not significantly changed when the plants were exposed to dark conditions (Supplementary Fig. S2c). Previous studies have indicated that the protein level of COP9 is not affected by light, but light may regulate the activity of the SlCSN5-2 protein at the posttranslational level43. Therefore, we believe that light is required for anthocyanin regulation in the SlBBX20-SlCSN5-2 model. These data reveal a novel regulatory pathway involved in light-induced anthocyanin biosynthesis.
Interestingly, when we knocked down the expression of SlCSN5 in tomato using RNAi, anthocyanins accumulated in the calli and shoots, and strongly silenced plants accumulated abundant anthocyanins but failed to grow into normal plants. A moderate decrease in the level of SlCSN5 expression led to dwarfing, indicating the important function of SlCSN5 in tomato development. Tomato contains two CSN5 genes, namely, SlCSN5-1 and SlCSN5-2. Because their sequences are highly similar, it is difficult to individually interfere with the expression of these genes. Indeed, previous studies also reported that it was impossible to use different sequences to separately silence the two CSN5 genes in tomato37. SlCSN5-VIGS plants were reported to be approximately 50% shorter in stature than controls37. The effects of SlCSN5 on growth and development may have been more severe in our stably transformed tomato lines. Among Arabidopsis thaliana, the csn5a mutant develops purple cotyledons44, but the reason is unknown. We found the sequence similarity between SlCSN5-2 and AtCSN5a to be 84%. We speculate that AtCSN5a utilizes a mechanism to regulate anthocyanin biosynthesis that is similar to that of SlCSN5-2.
CSN can regulate protein degradation through the ubiquitination pathway. The major activity of CSN is regulated by the fifth subunit (CSN5)45. CSN5 has been reported to be involved in deneddylation activity46. It can regulate the activity of CRLs (Cullin RING ligases) by covalently binding and removing RUB proteins47,48. Ubiquitinated proteins have been reported to accumulate in Arabidopsis csn mutants49. In the present study, we found that the ubiquitination of SlBBX20 was enhanced when it was coexpressed with SlCSN5. Furthermore, when we silenced the SlCSN5 homolog NbCSN5B in tobacco, accumulation of the SlBBX20 protein increased, indicating that CSN5 regulates accumulation of the SlBBX20 protein. Therefore, we infer that CSN5 is involved in ubiquitination and degradation of the SlBBX20 protein. Our previous study demonstrated that SlBBX20 can be ubiquitinated by the CUL4-DET1-DDB1 complex and eventually degraded by the 26 S proteasome34. CSN was found to modify the activity of several CUL4-based E3 ubiquitin ligases to regulate plant photomorphogenesis50. CSN5 might participate in regulating the activity of CUL4-based E3 ubiquitin ligases51. However, how CSN5 regulates the activity of CRLs and the accumulation of substrate remains unknown. Our work might provide insight into the modification of CSN5 to CRLs.
Materials and methods
Plant materials
The SlBBX20 gene was cloned into the overexpression vectors pHellsgate8 and pCAMBIA2300-HA. Using the “Alisa Craig” (LA2838A) tomato as the wild-type background, transgenic tomato plants were obtained by Agrobacterium tumefaciens-mediated transformation. The expression of SlBBX20 in the transgenic plants was quantified using qRT-PCR.
Gene expression analysis
TRIzol reagent (Invitrogen, USA) was used to extract total RNA from leaves as previously described52. cDNA was synthesized using a HiScript® II 1st Strand cDNA Synthesis Kit (Vazyme, China). Gene-specific oligonucleotides were used to perform qRT-PCR in a Roche LightCycler 480 system53. Relative gene expression was calculated by Microsoft Excel. Expression of the actin gene (SGN-U580609) was used as an internal control. The sequences of the gene-specific oligonucleotides used in the analysis are listed in Supplementary Table S2.
Measurement of the total anthocyanin content
The methanol-HCl method was used to extract anthocyanins from tomato leaves. Approximately 2 g of tomato leaves ground with liquid nitrogen was soaked in 5 ml of 1% (v/v) methanol HCl and extracted overnight in the dark at 24 °C. A spectrophotometer (UV-1600, Shimadzu, Japan) was used to measure the absorbance of each sample at 530, 620, and 650 nm. The following formula was used to calculate the relative anthocyanin content: optical density (OD) = (OD530-OD620)-0.1(OD650-OD620). One unit of anthocyanin represented a change in the OD of 0.1.
RNA sequencing
Three biological replicates from 4-week-old tomato seedlings that overexpressed SlBBX20 and WT tomato seedlings were selected for the extraction of total RNA and RNA sequencing. We used the average RPKM (reads per kilobase per million reads) value as a measure of gene expression54. Genes showing at least a twofold change in expression with an FDR-adjusted p-value of less than 0.05 were defined as differentially expressed genes (DEGs). The heat map was plotted with log2RPKM values to visually show differences in expression levels.
Yeast one-hybrid assay
The full-length SlBBX20 gene was amplified with tomato cDNA as the template and inserted into pGADT7 to obtain the prey vector (AD-SlBBX20). Fragments of the DFR, CHS1, CHS2, F3H, F3'5’H, and FLS promoters were amplified with tomato genomic DNA as the template and cloned into pAbAi to obtain a bait vector (pAbAi-DFR, CHS1, CHS2, F3H, F3'5’H, and FLS). The yeast strain Y1HGold transformed with bait vector was cultured on SD -Ura medium and placed in a 30 °C incubator for three days. Subsequently, the prey vector was transformed into the yeast strain Y1HGold previously transformed with the bait vector, and the resulting strain was plated on SD -Leu-Ura medium. The positive clones were diluted with 0.9% NaCl to an OD600 of 0.1, after which 2 μL of each suspension was spotted on SD -Leu medium with or without aureobasidin A (45 ng mL−1).
Transient dual-luciferase assay
The full-length SlBBX20 ORF was amplified by using tomato cDNA as a template and inserted into the pGreen II 62-SK vector. Promoter fragments from DFR (bp 1 to 1490) were cloned into the reporter vector, pGreen II 0800-LUC. The constructed vectors were individually introduced into Agrobacterium strain GV2260. The Agrobacterium liquid introduced into the reporter vector and effect vector were mixed and injected into tobacco leaves. Transient expression was evaluated three days after infiltration55. The firefly luciferase activity was detected by a dual-luciferase reporter assay system (Promega, USA).
Electrophoretic mobility shift assays (EMSAs)
The SlBBX20 gene was cloned into pET28a to express the His-tagged SlBBX20 protein. Based on the DFR promoter sequence, distinct 30-bp single-stranded fragments containing the cis-acting elements AACGTG or CACATGG were synthesized (TsingKe, China) and labeled using the Biotin 3′ End DNA Labeling Kit (Thermo Scientific, USA). The cis-acting element was replaced with a series of guanosines to obtain the mutated fragment. The labeled DNA fragment and purified His-SlBBX20 protein were incubated in the reaction mixture for 30 min as described previously18. The protein-DNA complexes were separated in 6.5% native PAGE gels. The gels were transferred to a nylon membrane (Beyotime Biotechnology, China). After UV cross-linking, chemiluminescence was used to observe the migration of the biotin-labeled probe on the membranes.
Yeast two-hybrid assay
The SlBBX20 coding sequence was inserted into the prey vector pGBKT7 (BD) to yield BD-SlBBX20, which was used as bait to screen a tomato yeast two-hybrid library, which showed that SlCSN5-2 and SlBBX20 interact. Furthermore, the coding sequence for a truncated SlCSN5-2 protein construct lacking 56 amino acids at its N-terminus (residues 57 to 367, SlCSN5-257-367) was amplified and cloned into the bait vector pGADT7 (AD) to yield AD-SlCSN5-2. The BD-S1BBX20 and AD-S1CSN5-2 plasmids were cotransformed into yeast strain AH109. After transformation, yeast AH109 cells were grown on SD -Trp-Leu medium for 3 days. A single clone was spotted in SD -Trp-Leu-His-Ade medium, and the growth of yeast cells was observed. The pGBKT7 and AD-SlCSN5-2 plasmids or BD-SlBBX20 and pGADT7 plasmids were used as negative controls.
Bimolecular fluorescence complementation (BiFC)
The SlBBX20 coding sequence was inserted into pHBT-nYFP to yield pHBT-SlBBX20-nYFP. The full-length SlCSN5-2 coding sequence was cloned into pHBT-cYFP to yield pHBT-SlCSN5-2-cYFP. Two plasmids, pHBT-S1BBX20-nYFP and pHBT-S1CSN5-2-cYFP, were cotransformed into Arabidopsis protoplasts. After the protoplasts had been cultured for 12 h, YFP fluorescence was observed by confocal microscopy.
Coimmunoprecipitation
For coimmunoprecipitation assays, tobacco protoplasts coexpressing SlBBX20-HA and SlCSN5-2-FLAG or expressing Mer as a control were collected, resuspended in extraction buffer and centrifuged at 12000 × g at 4 °C for 10 min. Five microliters of anti-FLAG matrix beads (Sigma, USA) were added to the supernatant and incubated at 4 °C for 2 h to capture the epitope-tagged protein. Finally, anti-HA (MBL, Japan) or anti-FLAG (Sigma, USA) antibodies were used for western blot analysis. Anti-UBQ (Millipore, USA) was used to detect ubiquitination of the SlBBX20 protein, and anti-actin was used as a control.
Subcellular localization
The SlCSN5-2 coding sequence without a stop codon was amplified and cloned into pHBT-GFP using gene-specific primers (Supplementary Tables S1). Tobacco protoplasts were extracted and cotransformed with the SlCSN5-2-GFP plasmid and a nuclear marker fused to RFP. After expression in protoplasts for 10 h, fluorescence was observed under a laser scanning confocal microscope.
Downregulation of CSN5 expression by virus-induced gene silencing (VIGS)
Because the two CSN5 sequences in tomato (CSN5-1 and CSN5-2) and two CSN5B sequences in tobacco (CSN5B-1 and CSN5B-2) are highly homologous, it is difficult to individually interfere with the two CSN5 sequences in a single species. Therefore, we elected to interfere with the expression of both. We employed virus-induced gene silencing (VIGS) and stable RNA interference (RNAi) to downregulate the expression of CSN5 in tobacco and tomato, respectively. For VIGS, we selected a unique sequence from NbCSN5B and ligated the sequence into pTRV2. The recombinant plasmid was transferred into Agrobacterium GV3101. Agrobacterium strains transformed with NbCSN5B-TRV2 and TRV1 or TRV2 and TRV1 were mixed and injected into tobacco leaves. After 10 days, the protoplasts were extracted from the tobacco, and SlBBX20-HA was transiently expressed. After SlBBX20-HA had been expressed in the protoplasts for 10 h, SlBBX20-HA was extracted and detected by western blotting. A unique sequence was selected from SlCSN5 and used to construct an RNAi vector for stable transformation. The sequences of the gene-specific oligonucleotides used in the analysis are listed in Supplementary Table S1.
Statistical analysis
Statistical analyses were performed using Prism 6 and SPSS 26.0. All of the experiments were repeated at least three times. Statistically significant differences were determined by subjecting the data to one-way ANOVA. The data are reported as the mean value ± SE. * indicates P < 0.05, and ** indicates P < 0.01.
References
Perez-Diaz, J. R. et al. New member of the R2R3-MYB transcription factors family in grapevine suppresses the anthocyanin accumulation in the flowers of transgenic tobacco. Plant Mol. Biol. 90, 63–76 (2016).
Fang, H. et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 42, 2090–2104 (2019).
An, J. P. et al. An Apple B-Box Protein MdBBX37 modulates anthocyanin biosynthesis and hypocotyl elongation synergistically with MdMYBs and MdHY5. Plant Cell Physiol. 61, 130–143 (2020).
An, J. P. et al. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation. Plant Biotechnol. J. 17, 2231–2233 (2019).
Bai, S. et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 17, 1985–1997 (2019).
Bai, S. et al. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 100, 1208–1223 (2019).
Peng, T. et al. Screening of UV-B-induced genes from apple peels by SSH: possible involvement of MdCOP1-mediated signaling cascade genes in anthocyanin accumulation. Physiol. Plant 148, 432–444 (2013).
Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 18, 477–483 (2013).
Yan, S. et al. Anthocyanin Fruit encodes an R2R3-MYB transcription factor, SlAN2-like, activating the transcription of SlMYBATV to fine-tune anthocyanin content in tomato fruit. N. Phytol. 225, 2048–2063 (2020).
Colanero, S., Tagliani, A., Perata, P. & Gonzali, S. Alternative splicing in the anthocyanin fruit gene encoding an R2R3 MYB transcription factor affects anthocyanin biosynthesis in tomato fruits. Plant Commun 1, (2020).
Sun, C. et al. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant 13, 42–58 (2020).
Zhang, Y., Butelli, E. & Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 19, 81–90 (2014).
Hichri, I. et al. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant 3, 509–523 (2010).
Kiferle, C. et al. Tomato R2R3-MYB proteins SlANT1 and SlAN2: same protein activity, different roles. PloS ONE 10, e0136365 (2015).
Jian, W. et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 6, 22 (2019).
Mathews, H. et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15, 1689–1703 (2003).
Espley, R. V. et al. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 49, 414–427 (2007).
Hu, D. et al. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol. 170, 1315–1330 (2016).
Wang, J. et al. Anthocyanin biosynthesis regulation in the fruit of Citrus sinensis cv. Tarocco. Plant Mol. Biol. Rep. 34, 1043–1055 (2016).
Xu, H. et al. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol. Biol. 94, 149–165 (2017).
Xie, X. B. et al. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 35, 1884–1897 (2012).
An, X., Tian, Y., Chen, K., Wang, X. & Hao, Y. The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. Plant Physiol. 169, 710–717 (2012).
Khanna, R. et al. The Arabidopsis B-box zinc finger family. Plant cell 21, 3416–3420 (2009).
Indorf, M., Cordero, J., Neuhaus, G. & Rodriguez-Franco, M. Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J. 51, 563–574 (2007).
Datta, S. et al. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant cell 20, 2324–2338 (2008).
Holtan, H. E. et al. BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol. 156, 2109–2123 (2011).
Fan, X. Y. et al. BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol. Plant 5, 591–600 (2012).
Gangappa, S. N. et al. The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 25, 1243–1257 (2013).
Wei, C. Q. et al. The Arabidopsis B-box protein BZS1/BBX20 interacts with HY5 and mediates strigolactone regulation of photomorphogenesis. J. Genet Genomics 43, 555–563 (2016).
Zhang, X. & Lin, R. Light signaling differentially regulates the expression of group IV of the B-box zinc finger family. Plant Signal Behav. 12, e1365213 (2017).
Yadav, A. et al. The B-Box-containing microprotein miP1a/BBX31 regulates photomorphogenesis and UV-B protection. Plant Physiol. 179, 1876–1892 (2019).
Ou, C. et al. A 14 nucleotide deletion mutation in the coding region of the PpBBX24 gene is associated with the red skin of “Zaosu Red” pear (Pyrus pyrifolia White Pear Group): a deletion in the PpBBX24 gene is associated with the red skin of pear. Hortic Res. 7, 39 (2020).
Fang, H. et al. MdCOL4 interaction mediates crosstalk between UV-B and high temperature to control fruit coloration in apple. Plant Cell Physiol. 60, 1055–1066 (2019).
Xiong, C. et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. N. Phytol. 221, 279–294 (2019).
Bursch, K. et al. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat. Plants 6, 921–928 (2020).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–427 (2002).
Hind, S. R. et al. The COP9 signalosome controls jasmonic acid synthesis and plant responses to herbivory and pathogens. Plant J. 65, 480–491 (2011).
Aharoni, A., Vos, C. H. R. D., Wein, M., Sun, Z. & O’Connell, A. P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 28, 319–332 (2010).
Albert, N. W., Davies, K. M., Lewis, D. H., Zhang, H. & Schwinn, K. E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell Environ. 26, 962–980 (2014).
Martin, C., Prescott, A., Mackay, S., Bartlett, J. & Vrijlandt, E. Control of anthocyanin biosynthesis in flowers of Antirrhinum majus. Plant J. 1, 37–49 (1991).
Kazama, Y. et al. Characterization of a heavy-ion induced white flower mutant of allotetraploid Nicotiana tabacum. Plant Cell Rep. 32, 11–19 (2013).
Chamovitz, D. A. et al. The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86, 115–121 (1996).
Wei, N., Daniel, A. C. & Deng, X. W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78, 117–124 (1994).
Gusmaroli, G., Figueroa, P., Serino, G. & Deng, X. W. Role of the MPN subunits in COP9 signalosome assembly and activity, and their regulatory interaction with Arabidopsis Cullin3-based E3 ligases. Plant cell 19, 564–581 (2007).
Jin, D., Li, B., Deng, X. W. & Wei, N. Plant COP9 signalosome subunit 5, CSN5. Plant Sci. 224, 54–61 (2014).
Wei, N. & Deng, X. W. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19, 261–286 (2003).
Wee, S., Geyer, R. K., Toda, T. & Wolf, D. A. CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat. Cell Biol. 7, 387–391 (2005).
Wei, N., Serino, G. & Deng, X. W. The COP9 signalosome: more than a protease. Trends Biochem Sci. 33, 592–600 (2008).
Peng, Z., Serino, G. & Deng, X. W. Molecular characterization of subunit 6 of the COP9 signalosome and its role in multifaceted developmental processes in Arabidopsis. Plant cell 13, 2393–2407 (2001).
Chen, H. et al. Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant cell 18, 1991–2004 (2006).
Biedermann, S. & Hellmann, H. WD40 and CUL4-based E3 ligases: lubricating all aspects of life. Trends Plant Sci. 16, 38–46 (2011).
Rio, D. C., Ares, M., Hannon, G. J. & Nilsen, T. W. Purification of RNA using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, pdb.prot5439-pd (2010).
Li, W. et al. Development and systematic validation of qPCR assays for rapid and reliable differentiation of Xylella fastidiosa strains causing citrus variegated chlorosis. J. Microbiol. Methods 92, 79–89 (2013).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13 (2005).
Acknowledgements
This work was supported by grants from the Fundamental Research Funds for the Central Universities (2662019PY048) and the National Natural Science Foundation of China (31772313, 31972421, and 31991182). We thank Professor Robert M. Larkin and Xiangzong Meng for critical reading of our manuscript.
Author information
Authors and Affiliations
Contributions
T.W., D.L., H.P. and Z.Y. conceived and designed the research. D.L., A.L., C.Z. and W.S. performed the experiments and carried out the fieldwork. D.L. and C.X. analyzed the data and wrote the manuscript. J.Z., C.Y., Y.L., H.L., H.P. and T.W. provided advice related to the research. All the authors have confirmed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, D., Xiong, C., Lin, A. et al. SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2 to regulate anthocyanin biosynthesis by activating SlDFR expression in tomato. Hortic Res 8, 163 (2021). https://doi.org/10.1038/s41438-021-00595-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41438-021-00595-y
This article is cited by
-
SlBBX28 positively regulates plant growth and flower number in an auxin-mediated manner in tomato
Plant Molecular Biology (2022)