Abstract
Inbreeding depression (ID) has since long been recognized as a significant factor in evolutionary biology. It is mainly the consequence of (partially) recessive deleterious mutations maintained by mutation-selection balance in large random mating populations. When population size is reduced, recessive alleles are increasingly found in homozygous condition due to drift and inbreeding and become more prone to selection. Particularly at slow rates of drift and inbreeding, selection will be more effective in purging such alleles, thereby reducing the amount of ID. Here we test assumptions of the efficiency of purging in relation to the inbreeding rate and the experimental conditions for four traits in D. melanogaster. We investigated the magnitude of ID for lines that were inbred to a similar level, F ≈ 0.50, reached either by three generations of full-sib mating (fast inbreeding), or by 12 consecutive generations with a small population size (slow inbreeding). This was done on two different food media. We observed significant ID for egg-to-adult viability and heat shock mortality, but only for egg-to-adult viability a significant part of the expressed inbreeding depression was effectively purged under slow inbreeding. For other traits like developmental time and starvation resistance, however, adaptation to the experimental and environmental conditions during inbreeding might affect the likelihood of purging to occur or being detected. We discuss factors that can affect the efficiency of purging and why empirical evidence for purging may be ambiguous.
Similar content being viewed by others
Introduction
When large populations become small, e.g., by destruction and fragmentation of natural habitats or during breeding programs, recessive deleterious alleles that were concealed in heterozygotes in the large population will become subjected to genetic drift, causing loss of genetic variation and fixation of alleles (Wright 1969; Hedrick 2005). If the level of drift is high enough, the frequency of these deleterious alleles can increase and even reach fixation, causing a permanent reduction in fitness (Charlesworth and Charlesworth 1987; Hedrick 1994; Glémin 2003; Lohr and Haag 2015). This fitness decrease will here be refered to as inbreeding depression (ID) as it is denoted in a conservation genetic context (Ehiobu et al. 1989; Bijlsma et al. 1999; Kristensen et al. 2011; Pekkala et al. 2014). In parallel with the appearance of ID, the relatedness among individuals in the small population will increase, even if the population is mating randomly, increasing the level of autozygosity (inbreeding coefficient F) (Wright 1969; Hedrick 2005). As such, a greatly reduced population size will (1) lead to a significant decline in fitness (Kimura 1983; Lynch et al. 1995), and (2) lead to an increase in the appearance of the deleterious alleles in homozygous state, where they become subjected to selection and eventually can be purged from the population (Hedrick 1994; Wang et al. 1999; Glémin 2003; García-Dorado 2012). The latter process (purging) is thus counteracting the loss of fitness (ID). Which of these processes does prevail depends strongly on the balance between the strength of selection (s), the degree of dominance of the deleterious allele (h), and the level of genetic drift that depends on the population size (Ne). Broadly, if Nes(1 − 2 h)/2 < 1 the effects of drift prevail and if Nes(1 − 2 h)/2 > 1 selection is the stronger factor (García-Dorado 2012; Lopez-Cortegano et al. 2016). It follows that when selection increases and/or the degree of dominance is reduced, purging will be more effective. More importantly, because the effectiveness of purging also depends on the population size, it follows that when populations of different sizes reach the same level of autozygosity, the balance between loss and fixation of deleterious mutations at the autozygous loci will differ. Hence, for the same level of inbreeding, a larger population with slower rate of inbreeding will lead to higher efficacy of purging ID compared to a smaller population with faster rate of inbreeding. Because ID is an important factor in many areas of biology, such as the evolution of mating systems and conservation genetics (Lynch and Walsh 1998), understanding the presence and dynamics of purging is of great importance.
Although theoretically the occurrence of purging through a slower inbreeding rate seems undisputed (Hedrick 1994; Wang et al. 1999; Glémin 2003; García-Dorado 2012), in practice the situation is much less clear. Experimental approaches that investigated the presence of purging sometimes support the idea (Ehiobu et al. 1989; Bijlsma et al. 2000; Pedersen et al. 2005; Swindell and Bouzat 2006a; Pekkala et al. 2014; Schou et al. 2017), while others show no or very little support (Frankham et al. 2001; Reed et al. 2003; Mikkelsen et al. 2010; Kristensen et al. 2011). Similarly, the empirical evidence is by no means unequivocal (Ballou 1997; Byers and Waller 1999; Crnokrak and Barrett 2002; Leberg and Firmin 2008).
In a sense, such varied results can be expected as the occurrence and magnitude of ID, and thus the possibility of purging to occur or to be detected, is affected by many factors. Among these: (1) traits differ widely in the amount of ID expressed upon inbreeding (Armbruster and Reed 2005; Angeloni et al. 2014) and therefore in their usefulness to signalize the process of purging. (2) The environment in which the inbreeding took place is crucial. Firstly, it determines which traits are expressed during inbreeding and which are not, and traits not expressed during inbreeding will not be subject to purging. Secondly, as the amount of ID is positively correlated with the stressfulness of the inbreeding environment (Armbruster and Reed, 2005; Cheptou and Donohue 2011; Fox and Reed 2011; Pedersen et al. 2011; Bijlsma and Loeschcke 2012), selection will be stronger in a stressful environment and therefore purging could be more effective. Consequently, the possibility and efficiency of purging may increase under more stressful conditions. (3) Similarly, the assay conditions under which the presence of purging is investigated are crucial. (4) Finally, adaptation to the experimental environmental conditions during (particular slow) inbreeding may be a confounding factor as the resulting increase in fitness due to adaptation may be incorrectly interpreted as purging (Willis 1999; Lopez-Cortegano et al. 2016). A number of other reasons can be found in Lopez-Cortegano et al. (2016).
In this paper, we investigate how different rates of genetic drift and inbreeding affect the magnitude of ID observed and test the influence of some of the factors mentioned above using D. melanogaster as a model organism.
We investigated the magnitude of ID of lines inbred to similar level, F ≈ 0.50, reached either by three generations of full-sib mating (fast inbreeding) or by 12 consecutive generations with a small population size of six pairs (slow inbreeding). The inbreeding procedure was carried out on two different food media: a nutrient rich medium and a nutrient poorer medium. The rationale for this was twofold. First, we assumed that the nutrient poorer medium was more stressful and, as such, affects the efficiency of purging compared to the richer medium (Lopez-Cortegano et al. 2016). Second, it was previously observed that ID detected on the poorer medium disappeared when the same inbred line was tested at the richer medium (Vermeulen and Bijlsma 2004; Vermeulen et al. 2008), suggesting the different media may generate different results. We studied this for two preadult fitness traits: egg-to-adult survival, a primary fitness trait, and developmental time, a trait more remotely related to fitness. In addition, we also studied two adult fitness traits: starvation resistance and heat resistance. These latter traits may not be under selection during the inbreeding procedure and therefore show no purging, unless these traits are pleiotropically affected by genes that are under selection during inbreeding (see Lopez-Cortegano et al. 2016). The experiments presented here aim to get a better insight into the relevance of purging for fragmented natural populations and its ecological and evolutionary consequences.
Materials and methods
Base population
The flies used for the experiments originated from the ODD base population that was founded with the offspring of more than 500 inseminated D. melanogaster females caught in September 2010 in a fruit orchard near Odder, Denmark (Schou et al. 2014). In the following generations, the flies were mixed each generation and maintained by placing 200 parents in each of five bottles. After 1.5 years the number of bottles was reduced to three bottles with 200 parents each. The base population was cultured at 25 °C on Leeds food medium (see next section) in a 12:12 h light/dark photoperiod. The experiments presented here were initiated ~3 years after the foundation of ODD.
Fly media
For the experiments, we made use of two different fly media. (1) Leeds (L) medium of which six liters of medium contained 300 g yeast 9 instant yeast, LeSaffre, Marq-en-Baroeul, France), 80 g agar, 200 g sugar, 150 g oatmeal, 60 ml Nipagin solution (10 g of Nipagin in 10 ml ethanol), and 6 ml acetic acid. (2) Groningen (G) medium of which six liters of medium contained 156 g yeast, 102 g agar, 324 g sugar, and 78 ml Nipagin solution. Both media were autoclaved before usage to prevent growth of microbes. Given that some 60–75% of oatmeal per weight consists of carbohydrates, the carbohydrate content, the energy source for the flies, of both media is more or less comparable. However, L-medium contains twice the amount of yeast, main source of proteins and (spore) elements, needed for growth. Therefore, we regard L-medium as far nutrient richer than G-medium and developmentally more suitable medium for larval growth.
Experimental lines
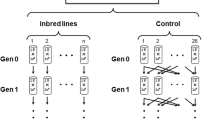
The lines used for the experiments were established by two different rates of inbreeding, which will be referred to as inbred lines in the paper. The fast inbred lines (F-lines) were made by full-sib mating. We started 50 vials with a single virgin female and a male from the base population for each of the two media used during inbreeding. Of the emerging offspring, one virgin female was collected and mated to a random brother. These brother–sister matings continued for three consecutive generations. A considerable number of the lines became extinct before the desired level of inbreeding was reached. In part, these extinctions may have been caused by low fitness due to inbreeding. A considerable part of it, however, is because we wanted to keep the lines synchronized on a strict 14-day generation cycle. Consequently, lines that did not produce females in the time interval(s) used for collecting virgins were also not included in the next generation. In the end, 15–20 lines from each inbreeding medium survived the three generations of inbreeding. From the remaining, with the exception of one or two lines that were so weak that they would have gone extinct before the experiments were finished, we randomly selected ten inbred lines from each inbreeding medium for our experiments. The slow inbred lines (S-lines) were established using a limited population size. In this case, we started 12 bottles each with six virgin females and six males from the base population and the next generation was established by collecting offspring: six virgin females that were mated to six randomly chosen males. This was done for each of the two food media and repeated for 12 consecutive generations. Based on experimental studies (Ehiobu et al. 1989; Nunney 1993), we assume that the genetically effective population size (Ne) is around 70% of the census size (although other estimates have been reported, varying from 59% (Lopez-Cortegano et al. 2016) to 81% (Pekkala et al. 2012)). Both the fast and slow inbreeding procedure will theoretically have reached a similar inbreeding coefficient of approximately F = 0.50. Because we wanted the inbred lines to have reached the desired level of inbreeding at the same time, we started the inbreeding procedure for the F-lines nine generations after the procedure of the S-lines had been started. In all these cases, we kept a constant generation time and the next generation was started 2 weeks after the previous.
During inbreeding, each of the two inbreeding procedures (F and S) was performed on both L- and G-medium resulting in four different combinations of inbreeding rate and inbreeding medium: FL, SL, FG, and SG. In the end, this resulted in ten independent inbred lines for each combination, thus a total of 40 lines. When the desired inbreeding level was reached for all inbred lines, we expanded the population size of the inbred lines and at the same time established ten independent Control (C) lines that were each started with some 100 randomly chosen flies from the base population.
Each of the 50 lines obtained in this way (10 C, 10 FL, 10 FG, 10 SL, 10 SG) was maintained in two bottles at 25 °C on L-medium for two generations prior to the experiments described below. Thus, the flies inbred on G-medium only experienced this specific food treatment during the inbreeding procedure prior to the experiments. Flies from the two replicate bottles were mixed each generation. The number of parental flies in each bottle was ~200 in each generation.
Experiments
Egg-to-adult viability
For each of the 50 lines, flies were allowed to lay eggs in bottles for 14 h. These eggs were thereafter picked to establish nine replicate vials with 40 eggs each on each of the two media for each line. In this way, a total of 36,000 eggs were transferred. These were incubated at 25 °C in vials with either 7 ml of L- or G-medium until the adults emerged. From these data, the egg-to-adult viability, being the fraction of eggs that resulted in adults, was calculated. Given the large number of eggs that had to be picked and transferred in 2 days, which is a tedious job, some counting errors or experimental errors, e.g., eggs because of static electricity of the plastic vial adhered to the plastic wall instead on the food and did not hatch, were to be expected. To this end, we decided to omit 24 of the 900 vials from the analysis, because they produced more than 42 adults (allowing for a small counting error of one or two eggs in surplus) and those with a suspiciously low progeny numbers (more than three standard deviations lower than the average of the nine replicates).
Developmental time
From the vials used for the egg-to-adult viability experiment, all adult flies were collected and counted every 12 h from the time of the first emerging flies until no flies eclosed anymore. From these data, developmental time, in hours from egg laying to eclosion was calculated as the median developmental time (DT50), the time point where 50% of the flies had eclosed per vial. To do so, we used linear interpolation of the eclosing percentages on time, in the time interval containing the 50% eclosion point.
Heat shock resistance
The experimental flies came from vials with controlled low egg numbers. For each of the 50 lines, six vials with ca. 70 eggs each were collected at two consecutive days with three vials per day and placed in vials with 7 ml of L-medium. When all flies had emerged ca. 11 days after egg collection, the flies in each vial were sexed and only males were used. Males were matured in vials with ten individuals per vial. Two days later ten males from each of the 50 lines were given a heat treatment in vials containing 3% plain agar medium in the bottom. The six replicates of each line were treated at two consecutive days (blocks). The males were heat stressed in a water bath at 37 °C for 2 h. After treatment, the vials were stored at room temperature to enable the flies to recover and the number of dead flies was recorded 6 h later. Heat stress mortality was calculated as the fraction of dead males.
Starvation resistance
The effect of starvation on pure agar medium was studied as time to death. For each of the 50 lines, 3 × 60 eggs were collected and placed in three vials with 7 ml L-medium. When all flies had eclosed, for each line flies from the three vials were mixed and sexed. Only males were used thereafter. The males were matured in vials at a density of ten.
Two days later 20 flies from each line were placed individually by aspirator in vials with 4 ml of plain agar medium (3% agar), giving a total of 1000 vials to score. The number of vials with a dead fly was recorded every 6 h and those vials were discarded after registration. The observations continued until the last fly died (after about 80 h). These data were used to estimate the time point, where 50% of the males had died (LT50) for each replicate line using linear interpolation in the time interval that contained the 50% mortality point. Males that died within the first 12 h of the test were regarded as accidental deaths and were not included in the calculations.
Statistical analyses
For each of the four phenotypic traits investigated we addressed two separate questions. First, we asked whether or not there was ID for the trait and, if so, which of the four inbreeding rates and inbreeding medium combinations were affected. This was done by performing pairwise comparisons between the control treatment and each of the inbreeding treatments (details and results can be found in Supplementary material: Text S1 and Tables S1–S4). Second, we investigated if the factors, Rate (F vs S) and Inbreeding medium (L vs G), affected the performance among inbred replicate populations. This was addressed by excluding the control treatment from the dataset and testing the effect of Rate, Inbreeding medium, their interaction and possibly additional trait-specific fixed effects. The significance of the fixed components was assessed with backward stepwise model reduction and likelihood ratio tests for mixed models or F-tests when no random effects were included (see below for details on model structure for each trait).
Egg-to-adult viability data were analyzed with generalized linear mixed models with a logistic link in the R package lme4 (Bates et al. 2014; R Core Team 2015), and contained the random effect Replicate line. For the analysis of differences among inbreeding treatments, we included the fixed effect Test medium, and the full set of interactions between this effect, Rate and Inbreeding medium.
In the analysis of developmental time, we estimated DT50 for each vial and used this as the response variable in linear mixed models. We used Replicate line as a random effect and included Sex as a fixed effect. To investigate the effect of Rate and Inbreeding medium and their dependence on the Test medium and Sex, we constructed a model with the full set of interactions between these effects.
The analysis of heat mortality was performed using generalized linear mixed models with a logistic link as for egg-to-adult viability. The models included the random effects Test replicate and Replicate line. For the analysis of starvation resistance, we estimated LT50 for each replicate line and used this as the response variable in the analysis of the trait using linear models.
We detected over-dispersion in the analysis of heat mortality and egg-to-adult viability and corrected this by including an observation level random effect in the models. All assumptions of parametric analysis were fulfilled.
Results
Egg-to-adult viability
Figure 1 depicts the average viability for each of the breeding treatments at both test media. We observed highly significant differences between the five breeding treatments both on Groningen (χ2(4) = 38.12, p < 0.001) and on Leeds (χ2(4) = 27.44, p < 0.001) test medium (see Table S1). On both media, the controls have a significantly higher viability than all inbreeding treatments, which signifies substantial ID for this trait. When we contrast the four inbreeding treatments, it is clear from Fig. 1 that the slow inbred lines have on average a much higher fitness than the fast inbred lines, which is confirmed by the statistical analysis (Table 1).
We can use the observed fitness values (Fig. 1) to estimate the inbreeding load B (number of lethal equivalents, see Hedrick and García-Dorado 2016) carried by the base population for the two inbreeding rates using the method of Morton et al. (1956) (see Table S2). On average B was found to be 0.132 for the four slow inbreeding treatments and 0.324 for the fast treatments, indicating that about 60% (range 40–70%) of the inbreeding load expressed under the fast scenario becomes purged under the slow scenario (Table S2).
When contrasting average egg-to-adult viability on the two test media, viability is significantly higher on L- than on G-medium (Fig. 1). Among the inbreeding treatments this results in a significant effect of the Test medium on survival (Table 1). So, viability is significantly higher on L-medium, however, this difference is on average <5%.
Developmental time
The results for developmental time are shown in Fig. 2. The most conspicuous observation is that development is much faster on L- than on G-medium, developing around 1 day faster (mean difference 24.49 h, SE = 0.53). Additionally, the differences in developmental time are also dependent on Rate of inbreeding, Inbreeding medium, and Test medium (Table 2). For all treatments, we also find that females develop slightly, but significantly, faster than males (Fig. 2 and Tables 2 and S3), a finding that is generally observed for D. melanogaster (Ashburner and Thompson 1978).
Comparison of the different breeding treatments shows that the control lines have the slowest development, while lines that were slowly inbred on G-medium (SG) show by far the fastest development, causing significant differences among Breeding treatments (Table S3). The difference between these two extremes is highly significant for both test media, while the remainder of the breeding treatments are significantly, or almost significantly, different from the controls as well.
Contrasting the inbreeding treatments reveals that Rate of inbreeding has a substantial impact on developmental time, with the slowly inbred lines developing on average 8.96 ± 2.26 h (mean ± SE) faster than the fast inbred ones (Table 2). However, this effect is mainly driven by the lines inbred on G-medium and is also dependent on other factors: Test medium, Inbreeding medium, and Sex (Table 2).
Heat shock mortality
For heat shock mortality, we observed significant differences among treatments that were mainly due to the control lines having a considerably lower mortality than the inbreeding treatments (Fig. 3), which was significant for all pairwise comparisons except for the Fast lines inbred on G-medium (Table S4). While this supports the presence of ID for this trait, we found no significant differences among inbreeding treatments driven by Rate of inbreeding or Inbreeding medium (Table 3).
Starvation resistance
Figure 4 shows several differences in average LT50 between the inbreeding media: lines inbred on G-medium seem more resistant than those inbred on L-medium. The resistance of the control lines falls in between these two. We found no evidence of ID as the fixed effect encompassing the five different treatments was non-significant (F(4,49) = 1.81; p = 0.14), and for this reason we did not proceed with pairwise comparisons to the control. However, the among inbreeding treatment analysis shows that there is a significant effect of Inbreeding medium: lines inbred on L-medium show a significant lower resistance than those inbred on G-medium (Table 4). There was no significant effect of Rate of inbreeding.
Discussion
The main question addressed in this paper is to what extent the efficiency of purging of ID mediated by a slower rate of inbreeding depends on the trait of study, the environmental conditions during inbreeding, and the test conditions, three factors that all have been reported to influence the efficiency of purging (Byers and Waller 1999; Crnokrak and Barrett 2002; Leberg and Firmin 2008).
The substantial ID observed for egg-to-adult viability (Fig. 1 and Tables 1 and S1) is not surprising, as this is a primary fitness trait. Preadult or juvenile survival have been generally observed to be negatively affected by small population size not only in D. melanogaster (Ehiobu et al. 1989; Armbruster and Reed 2005; Mikkelsen et al. 2010; Enders and Nunney 2016) but in most outcrossing organisms (Charlesworth and Charlesworth 1987; Charlesworth and Willis 2009). More importantly, the decrease in viability is significantly larger for the fast inbreeding treatment than for the slow inbreeding treatment (Fig. 1 and Table 1). This unequivocally shows that a significant part of the inbreeding load carried by the large non-inbred base population is purged under the slow inbreeding scenario. The number of lethal equivalents (Table S2) indicates that around 2/3 of the ID becomes purged under slow inbreeding. This value of 2/3 most likely concerns only the deleterious alleles, as the lethal and quasi-lethal alleles most probably have been purged under both inbreeding rates. This agrees well with other papers that have studied the effect of inbreeding rate in Drosophila both with respect to this trait (Ehiobu et al. 1989; Pedersen et al. 2005) and other primary fitness traits (Swindell and Bouzat 2006a, b; Bersabé and García-Dorado 2013; Pekkala et al. 2014). In conclusion, it shows that our experimental approach is effective in detecting the occurrence of purging, but several elements of uncertainty concerning the amount of ID purged need further attention.
Firstly, based on the biology of D. melanogaster, we assumed the Ne/N ratio to be 0.7 for the slow inbreeding rate. However, available estimates from experiments show that the ratio in fact may be considerably lower. Based on a microsatellite analysis Lopez-Cortegano et al. (2016) obtained an estimate of Ne/N = 0.52 for populations with a census size of N = 110, while Bakker (2008) using visible mutants estimated this ratio to be 0.56 for small populations of size N = 16. If the Ne/N ratio is indeed so much lower than we assumed, say 0.55, it will mean that the factual inbreeding coefficient would be 0.58 instead of 0.50. Correspondingly, the estimates for the number of lethal equivalents would decrease, meaning that the efficiency of purging in the slowly inbred lines most likely is underestimated rather than overestimated. While beyond the scope of this study, more precise estimates of the inbreeding level could have been obtained by using microsatellite or SNP analysis (Pekkala et al. 2012; Peripolli et al. 2017). However, several other factors such as selection may also have caused the realized inbreeding level to differ from the theoretically expected level, particularly around the loci causing the ID (Wang et al. 1999).
Secondly, during the fast inbreeding procedure we lost a considerable number of lines that in part would have been due to ID, thereby losing the most deleterious alleles. This means that the number of lethal equivalents carried by the base population revealed by this inbreeding rate is most likely underestimated. This idea is supported by the fact that our estimated range of B for the fast inbreeding rate (0.275–0.352) is considerably lower than the values generally observed for D. melanogaster populations, although the estimates vary greatly among populations. Simmons and Crow (1977) showed that the contribution of the second and third chromosome of D. melanogaster to the detrimental load, excluding lethals, was on average B = 0.520 (Lynch and Walsh 1998). Another explanation for our low estimates for B, however, is the possibility that this natural population from Denmark in the past has experienced regular low population sizes, for example during the winter periods, thereby loosing part of their deleterious load. This is one of the pitfalls when working with populations from the wild with unknown demographic/genetic history and may interfere with studies on purging in wild and captive populations (Ballou 1997; Byers and Waller 1999; Leberg and Firmin 2008).
In addition, we assumed that the expression of ID might depend on the environmental conditions (in this case different food sources). Indeed, the G-medium was more stressful and reduced the egg-to-adult viability by 1–5%, a small but significant amount, compared to the L-medium. Importantly, this was independent of the medium at which the inbreeding took place and of the inbreeding rate. Thus, the assumption that different food sources lead to differences in the amount of ID and the efficiency of purging is not validated in our experiment. Probably, the two inbreeding environments applied did not differ enough in stress level and we should either have reduced the nutritional value of the medium much more to evoke a sufficient level of stress (see Schou et al. 2015), or have applied a more divergent and stronger stress.
Notwithstanding some uncertainties in the extent of purging, the foregoing shows that the slow rate of inbreeding does promote purging. Thus, the next question is whether or not the other three traits follow the same pattern.
For developmental time, there are several intriguing results. Firstly, developmental time is much longer for the G-medium. Given the small difference in egg-to-adult viability, this is unlikely caused by a decreased fitness of the flies on this medium. We rather assume that it is either due to (1) its lower nutritional value forcing the larvae to spend a longer time eating for the same energy uptake as on the L-medium or (2) the larvae are slower eating because the G-medium is much firmer due to its higher agar content (Zwaan et al. 1991). Secondly, we observe that all inbreeding treatments develop faster than the non-inbred controls although the significance of the differences depends on other factors (Fig. 1 and Table S3). This is counterintuitive because physiological and/or metabolic impairment due to inbreeding is expected to prolong developmental time instead of shortening it (Bechsgaard et al. 2013). This is supported by several studies reporting an increase in developmental time due to inbreeding (Mikkelsen et al. 2010; Kristensen et al. 2011; Bechsgaard et al. 2013). Thus, for some reason ID was prevented to occur in our experiment. Thirdly, most main factors and many of their interactions significantly affected developmental time in our experiment (Table 2). The faster development of Drosophila females compared to males and the impact of different food sources on developmental time are well known (Ashburner and Thompson 1978; Zwaan et al. 1991). But given the absence of ID for this trait, a significant contribution of Rate of inbreeding is somewhat unexpected. However, close inspection of Fig. 3 and Table S3 reveals that particularly the flies from the slow inbreeding treatment on G-medium (SG-lines) show a strong decrease in developmental time compared to the other treatments. This is supported by the fact that Rate of inbreeding is highly significant in interaction with Test medium and Inbreeding medium, while Rate of inbreeding as a main effect is less pronounced (Table 2). How can we explain this? For the establishment of the inbred lines we had to collect virgin females and males from the offspring each generation to serve as parents for the next generation. Given that on G-medium development takes almost 1 day longer than on L-medium and we nevertheless wanted to synchronize the generation time for both inbreeding media, there is a need to start collecting virgins as soon as the first females eclose, particularly for the slower developing vials and bottles. Most likely, this has unintentionally resulted in selection for faster development. Although this will have affected all inbreeding treatments, it will have been by far the strongest for those lines developing on G-medium (slowest development) and inbred for 12 generations (SG). We, therefore, conclude that adaptation to the experimental conditions during inbreeding has prevented ID to occur for developmental time and interfered with our test for purging.
For heat shock mortality, we observed significantly increased mortality for heat stress upon inbreeding (Fig. 3). This is in line with the few studies that have previously investigated this trait: Dahlgaard et al. (1995) for D. buzzatii and Pedersen et al. (2005) for D. melanogaster also observed increased mortality for heat stress upon inbreeding. This is conceivable, because a decline of physical and biochemical functioning due to inbreeding may have consequences for heat stress resistance. However, despite the presence of ID, we did not observe a significant effect of Rate of inbreeding for this trait (Table 3). Therefore, differential purging for this trait did not occur. This suggests that the genes responsible for the loss of fitness for egg-to-adult viability do not show pleiotropic effects with respect to heat shock mortality nor for other traits (assayed or not) that been subject to purging under our inbreeding conditions. Our findings are in line with Pedersen et al. (2005) and Mikkelsen et al. (2010), who also observed that inbred lines of D. melanogaster had significantly lower heat stress resistance than outbred lines, but that this did not differ between the fast and slow inbreeding treatments. Similarly, Ehiobu et al. (1989) did observe ID for cold stress but no significant differences between slow and fast inbreeding treatments. The lack of purging can be explained by the lack of stress as a selective agent during the inbreeding procedure. Therefore, the deleterious effects of genes for stress resistance are not expressed at 25 °C. This view is supported by experiments with D. melanogaster, which showed that isogenic lines with normal fitness at 25 °C displayed very poor fitness at 29 °C (Dobzhansly and Levene 1955; Bijlsma et al.1999).
Starvation resistance did not show ID, as none of the inbreeding treatments differed significantly from the non-inbred control lines. The only other study directly testing the relation between inbreeding and starvation resistance in D. melanogaster is by Valtonen et al. (2011) who observed also no ID for this trait. A more indirect approach by Hoffmann et al. (2001) also supports our observation. In line with the absence of ID for this trait, the trait was not affected by the Rate of inbreeding nor by the interaction between Rate of inbreeding and Inbreeding medium (Table 4). However, we observed a significant effect of Inbreeding medium, with lines inbred on G-medium showing a significantly higher starvation resistance than those inbred on L-medium (Fig. 4 and Table 4). Starvation resistance is a complex trait affected by many ecological and evolutionary factors (Rion and Kawecki 2007) and it is suggested that a nutrition-restricted diet might result in increased adult starvation resistance through changes in the relative fat content of flies (Zwaan et al. 1991; Lee and Jang 2014; Kristensen et al. 2016). However, it has to be realized that all lines, including those that were inbred on G-medium, were maintained on L-medium a number of generations before this test was done. This suggests that the lines inbred on G-medium genetically adapted to this novel medium during inbreeding, which affected the response to inbreeding. This must have been a rapid response, because the fast inbred lines experienced the G-medium for only three generations. This could be explained by assuming that inbreeding not only exposes recessive deleterious alleles to selection but also low frequency recessive beneficial alleles. In this way, inbreeding could promote more rapid adaptation in small populations than in large ones. The findings of Swillen et al. (2015) that inbred lines of Daphnia outperformed outbred lines when inbreeding was done in the presence of insecticide stress supports this idea. The same phenomenon might explain why invasive species often rapidly adapt to new environments (Schrieber and Lachmuth 2017). It is clear that such adaptive processes during inbreeding will interfere with the possibility to detect both ID and purging for this trait. For instance, Willis (1999) showed that the improvement in fitness for an experimental plant population of Mimulus guttatis that was combined out of a large number of inbred lines was not due to purging but mostly the result of adaptation to the greenhouse conditions under which the plants were cultured. Adaptation to the environmental conditions might also interfere with other studies on purging, e.g., when studying captive populations (Ballou 1997).
In conclusion, detecting purging of ID is by no means straightforward. Our experiments show that detecting purging under a slow inbreeding rate is quite feasible for a primary fitness trait like egg-to-adult survival, which often shows high levels of ID independent of the environmental and experimental conditions. However, for other traits, the situation can be much more complex and many factors can interfere with the purging process. Adaptation to the experimental and environmental conditions during inbreeding might affect the amount of ID expressed and thus the likelihood of purging to occur or being detected, like in this case for developmental time and starvation resistance. Moreover, traits not only have to show ID but should also be under selection during inbreeding, otherwise differential purging will not occur, like we observed for heat shock resistance. Given the forgoing, it is, therefore, not surprising that the empirical and experimental evidence for purging to occur is ambiguous. Much more research is needed to evaluate consequences of inbreeding environment and assay environment for the efficiency of purging, if we are to increase our understanding of the ecological and evolutionary consequences of purging in natural populations.
Data availability
All data presented in the paper are deposited in Dryad https://doi.org/10.5061/dryad.rfj6q579k.
References
Angeloni F, Vergeer P, Wagemaker CAM, Ouborg NJ (2014) Within and between population variation in inbreeding depression in the locally threatened perennial Scabiosa columbaria. Conserv Genet 15:331–342
Armbruster P, Reed DH (2005) Inbreeding depression in benign and stressful environments. Heredity 95:235–242
Ashburner M, Thompson JN (1978) The laboratory culture of Drosophila. In: Ashburner M, Wright TRF (eds) The Genetics and Biology of Drosophila, vol. 2a. Academic Press, NewYork, NY, p 2–109
Bakker J (2008) Genetic diversity in experimental metapopulations. Ph.D. thesis, University of Groningen, The Netherlands
Ballou JD (1997) Ancestral inbreeding only minimally affects inbreeding depression in mammalian populations. J Heredity 88:169–178
Bates D, Maechler M, Bolker B, Walker S (2014). lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-5. http://cran.r-project.org/package=lme4
Bechsgaard JS, Hoffmann AA, Sgró C, Loeschcke V, Bilde T, Kristensen TN (2013) A comparison of inbreeding depression in tropical and widespread Drosophila species. PLoS ONE 8:e51176
Bersabé D, García-Dorado A (2013) On the genetic parameter determining the efficiency of purging: an estimate for Drosophila egg-to-pupae viability. J Evol Biol 26:375–385
Bijlsma R, Bundgaard J, Van Putten WF (1999) Environmental dependence of inbreeding depression and purging in Drosophila melanogaster. J Evol Biol 12:1125–1137
Bijlsma R, Bundgaard J, Boerema AC (2000) Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. J Evol Biol 13:502–514
Bijlsma R, Loeschcke V (2012) Genetic erosion impedes adaptive responses to stressful environments. Evol Appl 5:117–129
Byers D, Waller D (1999) Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu Rev Ecol Syst 30:479–513
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Cheptou PO, Donohue K (2011) Environment-depend inbreeding depression: its ecological and evolutionary significance. N Phytol 189:395–407
Crnokrak P, Barrett SCH (2002) Perspective: purging the genetic load: a review of the experimental evidence. Evolution 56:2347–2358
Dahlgaard J, Krebs RA, Loeschcke V (1995) Heat-shock tolerance and inbreeding in Drosophila buzzatii. Heredity 74:157–163
Dobzhansly T, Levene H (1955) Genetics of natural populations. XXIV. Developmental homeostasis in natural populations of Drosophila pseudoobscura. Genetics 40:797–808
Ehiobu NG, Goddard ME, Taylor JF (1989) Effect of rate of inbreeding on inbreeding depression in Drosophila melanogaster. Theor Appl Genet 77:123–127
Enders LS, Nunney L (2016) Reduction in the cumulative effects of stress-induced inbreeding depression due to intragenerational purging in Drosophila melanogaster. Heredity 116:304–313
Fox CW, Reed DH (2011) Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65:246–258
Frankham R, Gilligan DM, Morris D, Briscoe DA (2001) Inbreeding and extinction: effects of purging. Conserv Genet 2:279–285
García-Dorado A (2012) Understanding and predicting the fitness decline of shrunk populations: inbreeding, purging, mutation, and standard selection. Genetics 190:1461–1476
Glémin S (2003) How are deleterious mutations purged? Drift versus nonrandom mating. Evolution 57:2678–2687
Hedrick PW (1994) Purging inbreeding depression and the probability of extinction—full-sib mating. Heredity 73:363–372
Hedrick PW (2005). Genetics of populations, 3rd edn. Jones and Bartlett Publishers, Sudbury, MA, p 368
Hedrick PW, García-Dorado A (2016) Understanding inbreeding depression, purging, and genetic rescue. Trends Ecol Evol 31:940–952
Hoffmann AA, Hallas R, Sinclair C, Mitrovski P (2001) Levels of variation in stress resistance in Drosophila among strains, population, and geographic regions: patterns for desiccation, starvation, cold resistance, and associated traits. Evolution 55:1621–163
Kimura M (1983) The neutral theory of molecular evolution. Cambridge University Press, Cambridge
Kristensen TN, Knudsen MR, Loeschcke V (2011) Slow inbred lines of Drosophila melanogaster express as much inbreeding depression as fast inbred lines under semi-natural conditions. Genetica 139:441–451
Kristensen TN, Henningsen AK, Aastrup C, Bech-Hansen B, Hoberg Bjerre LB, Carlsen B et al. (2016) Fitness components of Drosophila melanogaster developed on a standard laboratory diet or a typical natural food source. Insect Sci 23:771–779
Leberg PL, Firmin BD (2008) Role of inbreeding depression and purging in captive breeding and restoration programmes. Mol Ecol 17:334–343
Lee KP, Jang T (2014) Exploring the nutritional basis for starvation resistance in Drosophila melanogaster. Funct Ecol 28:1144–1155
Lohr JN, Haag CR (2015) Genetic load, inbreeding depression, and hybrid vigor covary with population size: an empirical evaluation of theoretical predictions. Evolution 69:3109–3122
Lopez-Cortegano E, Vilas A, Caballero A, García-Dorado A (2016) Estimation of genetic purging under competitive conditions. Evolution 70:1856–1870
Lynch M, Conery J, Buerger R (1995) Mutation accumulation and the extinction of small populations. Am Nat 146:489–518
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits, chapter 10. Sinnauer Associates: Sunderland, MA
Mikkelsen K, Loeschcke V, Kristensen TN (2010) Trait specific consequences of fast and slow inbreeding: lessons from captive populations of Drosophila melanogaster. Conserv Genet 11:479–488
Morton NE, Crow JF, Muller HJ (1956) An estimate of the mutational damage in man from data on consanguineous marriages. Proc Nat Acad Sci USA 42:855–863
Nunney L (1993) The influence of mating system and overlapping generations on effective population-size. Evolution 47:1329–1341
Pedersen KS, Kristensen TN, Loeschcke V (2005) Effect of inbreeding and rate of inbreeding in Drosophila melanogaster—Hsp70 expression and fitness. J Evol Biol 18:756–762
Pedersen LD, Pedersen AR, Bijlsma R, Bundgaard J (2011) The effects of inbreeding and heat stress on male sterility in Drosophila melanogaster. Bio J Linn Soc 104:432–442
Pekkala N, Knott KE, Kotiaho JS, Puurtinen M (2012) Inbreeding rates modifies the dynamics of genetic load in small populations. Ecol Evol 2:1791–1804
Pekkala N, Knott KE, Kotiaho JS, Nissinen K, Puurtinen M (2014) The effect of inbreeding rate on fitness, inbreeding depression and heterosis over a range of inbreeding coefficients. Evol Appl 7:1107–1119
Peripolli E, Munari DP, Silva MVGB, Lima ALF, Irgangand R, Baldi F (2017) Runs of homozygosity: current knowledge and applications in livestock. Anim Genet 48:255–271
R Core Team (2015) R: a language and environment for statistical computing. http://www.r-project.org/
Reed DH, Lowe EH, Briscoe DA, Frankham R (2003) Inbreeding and extinction: effects of rate of inbreeding. Conserv Genet 3:405–410
Rion S, Kawecki TJ (2007) Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol 20:1655–1664
Schou MF, Kristensen TN, Kellermann V, Schlötterer C, Loeschcke V (2014) Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. J Evol Biol 27:1859–1868
Schou MF, Loeschcke V, Kristensen TN (2015) Inbreeding depression across a nutritional stress continuum. Heredity 115:56–62
Schou MF, Loeschcke V, Schlötterer C, Bechsgaard J, Kristensen TN (2017). Unexpected high genetic diversity in small populations suggests maintenance by associative overdominance. Mol Ecol (in press). https://doi.org/10.1111/mec.14262
Schrieber K, Lachmuth S (2017) The genetic paradox of invasions revisited: the potential role of inbreeding x environment interactions in invasion success. Biol Rev 29:939–952
Simmons MJ, Crow JF (1977) Mutations affecting fitness in Drosophila populations. Ann Rev Genet 11:49–78
Swillen I, Vanoverbeke J, De Meester L (2015) Evolution of carbaryl resistance in the water flea Daphnia: complex interactions between inbreeding, stress, and selection. Hydrobiologica 743:199–209
Swindel WR, Bouzat JL (2006a) Selection and inbreeding depression: effects of inbreeding rate and inbreeding environment. Evolution 60:1014–1022
Swindell WR, Bouzat JL (2006b) Ancestral inbreeding reduces the magnitude of inbreeding depression in Drosophila melanogaster. Evolution 60:762–766
Valtonen TM, Roff DA, Rantala MJ (2011) Analysis of the effects of inbreeding on lifespan and starvation resistance in Drosophila melanogaster. Genetica 139:525–533
Vermeulen CJ, Bijlsma R (2004) Changes in mortality patterns and temperature dependence of lifespan in Drosophila melanogaster caused by inbreeding. Heredity 92:275–281
Vermeulen CJ, Bijlsma R, Loeschcke V (2008) QTL mapping of inbreeding-related cold sensitivity and conditional lethality in Drosophila melanogaster. J Evol Biol 21:1236–1244
Wang JL, Hill WG, Charlesworth D, Charlesworth B (1999) Dynamics of inbreeding depression due to deleterious mutations in small populations: mutation parameters and inbreeding rate. Genet Res 74:165–178
Willis JH (1999) The role of genes of large effect on inbreeding depression in Mimilus gutatus. Evolution 53:1678–1691
Wright S (1969) Evolution and the genetics of populations. Vol. 2, The theory of genetic frequencies. University of Chicago Press, Chicago and London
Zwaan BJ, Bijlsma R, Hoekstra RF (1991) On the developmental theory of ageing. 1. Starvation resistance and longevity in Drosophila melanogaster in relation to pre-adult breeding conditions. Heredity 66:29–39
Acknowledgements
We are grateful to Doth Andersen and Annemarie Højmark for technical help, and the Danish Natural Sciences Research Council (FNU, grant 4002-00113B) for financial support to VL. We thank three reviewers for their constructive comments on an earlier version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Darren Obbard
Supplementary information
Rights and permissions
About this article
Cite this article
Bundgaard, J., Loeschcke, V., Schou, M.F. et al. Detecting purging of inbreeding depression by a slow rate of inbreeding for various traits: the impact of environmental and experimental conditions. Heredity 127, 10–20 (2021). https://doi.org/10.1038/s41437-021-00436-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41437-021-00436-7
This article is cited by
-
Thermal Stress and Adult Fitness in a Drosophila suzukii Neotropical Propagule
Neotropical Entomology (2023)
-
Genetic purging in captive endangered ungulates with extremely low effective population sizes
Heredity (2021)