Abstract
Purpose
As genomic screening is incorporated into a wider array of clinical settings, it is critical that we understand how patients may respond to a various screening results. Although multiple studies have examined how patients understand positive genomic screening results, few data exist regarding patient engagement with negative screening results.
Methods
An 82-item survey was administered to 1712 individuals who received negative genomic screening results by mail following evaluation of 109 medically actionable genes. Genetic counselors were available to assist with the interpretation of screening results.
Results
One thousand four hundred forty-two participants completed the survey (84.2%). The vast majority of respondents valued the information they received, with 98% of respondents reporting that negative genomic screening results were valuable and 72% indicating they would recommend genomic screening to others. Nonetheless, many respondents had questions about their genomic screening results (28%) and would have preferred to receive their screening results in person (18%).
Conclusion
These data suggest most patients value receiving negative genomic screening results and are comfortable receiving their results by mail. Nevertheless, a significant proportion of patients also reported difficulty understanding some aspects of their results. This finding challenges the idea that communicating genomic screening results by mail alone is sufficient to meet patients’ needs.
Similar content being viewed by others
INTRODUCTION
Tens of thousands of individuals are offered some type of genomic screening each year.1 Although the hope is that patients with undetected genetic risk factors will be identified through these efforts, the vast majority of these individuals will receive a finding that does not suggest a need for any behavioral change or medical intervention.2 It is unclear to what extent patients value receiving these types of results, often called “negative” genomic screening results.3 Given the labor-intensiveness and cost associated with returning negative results from large scale screening, it is important to evaluate whether and how much patients value receiving such results to address questions of whether negative results should be returned. In addition, it is unclear how patients may understand the medical implications of a negative genomic screening result given the many misconceptions surrounding the predictive power of genetic information.4
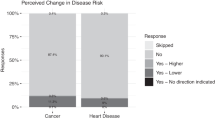
Of particular concern is the possibility that patients may draw inappropriate conclusions about their likelihood of developing a health condition in the future or may be less likely to adopt risk reduction strategies based on their interpretation of negative genomic screening results. These concerns are especially salient given the potential ambiguity of a negative genomic screening result. While such results could imply that no genetic risk factors are present, negative findings should be interpreted with caution in light of current limitations with reference data sets and other knowledge gaps in our understanding of variant pathogenicity.5 These interpretive nuances may be difficult to convey to patients in brief communications, resulting in some patients declining to pursue other medically appropriate screening or preventive interventions as a result of a mistaken belief that they have a “clean bill of health” are not at risk of developing disease.
Currently, very few data are available describing how patients engage with negative genomic screening results, especially when those results are reported outside of in-person genetic counseling sessions. Although prior studies have examined the effectiveness of different methods of returning genomic results,6,7,8,9 these studies have focused largely on the reporting of positive screening results or variants of uncertain significance (VUS). Additionally, few studies have examined the effectiveness of communication approaches that do not involve reporting genomic screening results through a genetic counselor or other trained heath professionals.10,11
This study examined patient experiences receiving negative genomic screening results by mail, including patient understandings of these results and their assessments of the value of a negative genomic screening result. This work is well-timed considering the rapid expansion of genomic screening12 and large numbers of patients who will receive a negative screening result in the future. The results we report can inform the design of scalable alternatives to reporting genomic results to patients during an in-person genetic counseling appointment.
MATERIALS AND METHODS
Ethics statement
This study was approved by the Mayo Clinic IRB #15-005013. Informed consent was obtained from all participants. This study adhered to the principles set out in the Declaration of Helsinki.
Setting and participants
We surveyed individuals receiving negative genomic screening results in the context of a large genomic implementation study. Though genomic sequencing was a clinical test, the study we report here is a translational research study designed to simulate the contexts in which patients might be offered genomic screening in the future. Our findings apply to both the research and clinical settings due to the nature of the study. Participants of this study were members of the Mayo Clinic biobank who had a phenotype of hyperlipidemia and/or colon polyps. This study was part of the eMERGE consortium funded by the US National Institutes of Health and provided genomic sequencing of 109 medically actionable genes.13 To participate in the study, participants must have been willing to receive their results. Pretest genetic counseling was available to participants at no cost but was not a requirement for participation.14 A study flow diagram can be found in Kullo et al.13
Clinical laboratory reports were generated for each study participant and placed in participants’ electronic medical record. Genetic results suggesting a need for medical follow up (i.e., “positive screening results”) were communicated to participants in person or by telephone by a licensed genetic counselor. Genomic results that did not have any known health implications (i.e., “negative screening results”) were returned to patients by postal mail. All participants received results between 21 and 25 months after the time of consent. Materials included in this mailed communication included a one-page letter summarizing test results (available upon request) and a copy of the laboratory report that was entered into the patient’s electronic medical record. The letter explained the results, the limitations of the results, and recommended participants share the result with their provider. The laboratory report included each gene listed with a brief description. Variants of uncertain significance were not returned. Free genetic counseling support was also available to participants who had questions about their screening results, and participants were informed of this support in the results letter, which also listed a phone number for accessing such services.
Survey
We developed an 82-item survey consisting mostly of de novo items developed by the research team. Survey items examined motivations for pursuing genomic screening, expectations about screening results, initial reactions to receiving negative genomic screening results, self-reported comprehension of screening results, perceived value of screening results, perceived risk of disease, familiarity with family history, previous experiences with genetic testing, plans to share screening results with others, and overall reflections on the experience of genomic screening.
Participants who received negative genomic screening results from the 109-gene panel had previously completed a baseline psychosocial questionnaire following their initial enrollment in the genomic implementation study.15 This survey collected demographic information on participants, as well as data on multiple independent variables of interest, including health literacy, health-care access, financial stability, insurance coverage, and knowledge about genomic sequencing. Not all survey data are presented in this paper.
Data collection
This study was approved by the Mayo Clinic Institutional Review Board (#15-005013). Surveys were mailed to participants approximately 14 days after they received the letter and lab report informing them of their negative genomic screening result. Screening results were mailed to participants beginning in April 2018, with the first wave of surveys following in early May 2018. Nonresponders to the survey received a reminder and an additional survey approximately 30 days after the first mailing.
Trained staff from the Mayo Clinic Survey Research Center monitored survey completion. Each returned survey was date-stamped and documented in a tracking database. Data from completed surveys were doubled-entered by data entry staff, and research staff conducted periodic quality checks. Individual responses to survey items that were unclear to data entry personnel were flagged and the paper surveys reviewed by a research team member. Flagged responses for which participant intent could be reasonably ascertained by research staff were updated in the data set; ambiguous responses were marked as missing.
Data analysis
Data were analyzed using JMP Pro 14 (2018 SAS Institute Inc). Means, medians, standard deviations, and ranges were calculated for continuous variables, and frequencies and percentages were calculated for categorical variables. Bivariate associations were calculated using chi-square, Wilcoxon rank sum, and Fisher's exact test, as appropriate. P values of 0.05 or lower were considered statistically significant.
The primary outcomes of interest for the analysis reported here are (1) patient interest in receiving negative genomic screening results, (2) patient reactions to receiving negative genomic screening results by mail, and (3) patient perceived understanding of results. Patient interest in receiving negative genomic screening results was assessed by responses to the survey item “How valuable was it to learn that you have no genetic variants indicating increased risk of disease?” answered with the response options of Extremely Valuable, Quite Valuable, Slightly Valuable, and Not At All Valuable. Patient reactions to receiving negative genomic screening results by mail were assessed by three survey items answered on a 5-point Likert scale of agreement (Strongly Agree, Agree, Neither Agree Nor Disagree, Disagree, Strongly Disagree): (1) “I would go for the same choice to participate in the RAVE study if I had to do it over again,” (2) “I would recommend the genetic testing I received as part of the RAVE study to my friends and family,” and (3) “I wish I had received my genetic test results in person.” Patient reactions were also assessed by the following two survey items assessing disappointment, which had the following response options: Very Disappointed, Somewhat Disappointed, and Not Disappointed At All: (1) “How disappointed were you that researchers did not find in your DNA any genetic variants that indicate increased risk of disease” and (2) “How disappointed were you that researchers did not find in your DNA any genetic variants that contribute to a disease you already have.” Patient perceived understanding of results was assessed by responses to the following three survey items answered on a 5-point Likert scale of agreement (Strongly Agree, Agree, Neither Agree Nor Disagree, Disagree, Strongly Disagree): (1) “When I first read the letter describing my test results, it was difficult to understand,” (2) “I felt the lab report was difficult to understand,” and (3) “I still have questions about what my genetic test results mean.”
Genetic knowledge scores were computed by summing correct responses to an 11-item measure administered at baseline that was developed and published by another research group.16 Responses were answered on a 5-point Likert scale of agreement (Strongly Agree, Agree, Neither Agree Nor Disagree, Disagree, Strongly Disagree). Illustrative questions include “Genome sequencing may find variants in a person’s genes that will increase their chance of developing a disease in their lifetime” and “Even if a person has a variant in a gene that affects their risk of a disease, they may not develop that disease.”
Familiarity with study procedures scores were computed by summing correct responses to nine knowledge questions with response options of True, False, and I Do Not Know. Illustrative questions include “My genetic test results from the RAVE study have been placed in my electronic health record” and “The genetic testing done as part of the RAVE study cannot detect all genetic variants that may eventually be known to cause disease.” Missing data for individual items were scored as “incorrect.”
Difficulty understanding results scores were computed from responses to the following three survey items answered on a 5-point Likert scale of agreement (Strongly Agree, Agree, Neither Agree Nor Disagree, Disagree, Strongly Disagree): (1) “When I first read the letter describing my test results, it was difficult to understand,” (2) “I felt the lab report was difficult to understand,” and (3) “I still have questions about what my genetic test results mean.”
Questions answered on a 5-point Likert scale were dichotomized Agree against Not Agree/Disagree. If they did not clearly agree with the statement (i.e., the Neither Agree nor Disagree option), we took them as not agreeing and lumped them in with those who disagreed.
To standardize the difficulty understanding results score with the familiarity with study procedures score described above (range of 0 to 9), we scored agreement (Strongly Agree/Agree) to each perception question as 0, neutral responses (Neither Agree Nor Disagree) were coded as 1.5, and disagreement (Disagree/Strongly Disagree) was coded as 3. Recoded scores for each question were summed, resulting in a single score of difficulty understanding results ranging from 0 to 9.
RESULTS
Of the 5110 participants who met eligibility criteria and were invited to participate in the genomic implementation study, 2538 responded to the study invitation, consented to participate, and elected to pursue genomic screening.13 Of the 5110 individuals who were invited to participate, only 8 individuals elected to have pretest genetic counseling.13 A total of 118 individuals (4.6%) received a “positive” genomic screening result and 2416 individuals (95.2%) were informed that no clinically actionable results were found (4 individuals withdrew from the study). Although genetic counseling services were available to participants who had questions about their results at no cost, just four participants requested to speak with a genetic counselor after receiving a negative genomic screening result.
After a first pilot cohort of negative result recipients received their results, a mailed survey was sent to the remaining 1712 individuals who received a negative genomic screening result and had previously completed the baseline demographic survey. Of these individuals, 1442 completed the survey (84.2% completion rate). Table 1 summarizes demographic characteristics of individuals who pursued genomic screening and completed the survey after receiving a negative result. Our sample was comprised largely of white (96.5%) and older (mean age = 60.8) individuals, with more women (57.6%) represented than men (42.2%). We include demographic variables in each table because we sought to assess potential associations between demographic variables and specific patient responses to receiving genomic screening results by mail.
Table 2 compares respondent demographics across variables indicative of a favorable experience receiving genomic screening results by mail. Ninety-eight percent of individuals reported that it was valuable to learn that they have no genetic variants indicating increased risk of disease. Nearly all respondents indicated that they found their results valuable (98.0%) and indicated that they would participate in the study again (95.1%). Most reported that they would recommend this type of screening to others (72.2%). Individuals who reported at least one favorable experience were more likely to have higher genetic knowledge, more familiarity with study procedures, and less difficulty understanding results. Individuals who reported favorable experiences were also more likely to have adequate health literacy and private insurance coverage.
Of note, higher genetic knowledge scores, higher levels of education, and adequate health literacy were associated with individuals reporting they would participate in the study again, but were not associated with reporting that the results received were valuable or with recommending study participation to others. Those with private insurance coverage were also more likely to recommend the study to others, though insurance coverage was not associated with individuals reporting the results received were valuable or with individuals reporting they would participate in the study again. Sex, age, race, ethnicity, marital status, inability to access a physician due to cost, and income were not statistically associated with any favorable responses.
Table 3 summarizes demographic characteristics across survey items assessing patient dissatisfaction with receiving negative genomic screening results. In our sample, 12.6% of individuals were disappointed with results and 17.8% would have preferred that their genomic screening results were returned in person. Individuals who were dissatisfied with the results they received and those who would have preferred that their results be returned in person were more likely to have lower genetic knowledge and higher difficulty understanding results scores. Individuals who were dissatisfied with results were more likely to have less education, inadequate health literacy, less income, and be insured through a public program. While age, lower levels of education, less income, and insurance coverage through a public program were associated with individuals reporting that they would have preferred to receive their genomic screening results in person, these variables were not associated with individuals reporting that they were disappointed with their results. Sex, race, ethnicity, marital status, inability to access a physician due to cost, and income were not statistically associated with any unfavorable responses.
Finally, Table 4 presents results on perceived understanding of negative genomic screening results across sample demographics. A total of 29.5% of individuals reported that the letter was difficult to understand, 46.6% of individuals reported the lab report was difficult to understand, and 28.0% reported that the results left them with questions. Individuals who reported difficulty understanding the letter, difficulty understanding the lab report, and who had unanswered questions about their results were more likely to be older and have lower genetic knowledge. Additionally, individuals who reported that the letter was difficult to understand were more likely to have lower levels of education, lower health literacy, less income, and be insured by a public program. Individuals who reported that the lab report was difficult to understand were also more likely to be insured by a public program.
DISCUSSION
This is the first study to examine patient experiences receiving negative genomic screening results by mail. Our results support four broad findings that should inform the development of future genomic screening activities.
First, our data offer clear evidence that patients desire and value receiving negative genomic screening results. The vast majority of patients reported value in receiving negative screening results and were satisfied with their overall experience. Although negative screening results are typically returned in diagnostic settings, there is considerable diversity regarding whether and how negative genetic test results are returned in research settings.17 A limited genetic counseling workforce and rapidly growing need for counseling services combine to challenge the practicality of returning genomic screening results that have limited implications for ongoing care or future health.18,19 Given the limited availability of licensed genetic counselors, prioritization of counseling services might focus on the reporting of medically actionable findings, particularly results that may be difficult to interpret.14 Our data suggest that alternative communication strategies may need to be developed to meet the needs of patients, many of whom will want to receive their genomic screening results, even when those results may not require a medical response.
Second, in contrast to various concerns that have been voiced about the disruptive potential of genomic screening to the health-care system,20,21,22 or in particular, the potential of genomic screening to place a large burden on genetic counselors and other providers to answer patient questions about results, we did not find evidence of widespread disruption with the communication of negative genomic screening results by mail. Very few patients requested assistance from a genetic counselor in the interpretation of their results. Our data suggest that reporting negative genomic screening results by mail is unlikely to trigger widespread disruptions in the health-care system.
Third, although patient interest in receiving negative screening results was high and very few patients requested assistance from a genetic counselor in interpreting their results, many patients reported difficulty understanding the letter they received and indicated that they had unanswered questions about their results. It is noteworthy that approximately half of all participants reported difficulty understanding the clinical laboratory report they received.
These data suggest that the inclusion of a clinical laboratory lab report may contribute to more difficulty for patients to understand negative genomic screening results. One option is for such reports to be made available to those who are interested but not routinely included in the communication of negative screening results. In other settings, nongenetic laboratory reports have been shown to cause confusion and anxiety and may not be provided to patients routinely in some health systems.23 Alternatively, it may be helpful to develop ancillary patient support materials that focus on the interpretation of the clinical laboratory report. These ancillary materials could take many forms and might include a frequently asked questions document or web-based interfaces, which have been shown to be effective in improving patient comprehension of genetic results in other contexts.11,16 These materials could provide additional information about specific types of screening results, clarify the implications of genomic screening results for other health screening services, and clarify how patients who wish to follow up with a medical professional should proceed. To be clear, we are not suggesting that our data indicate withholding the lab report. Rather, our findings support that if you return the lab report, doing so responsibly would mean including other materials. The letter used here did not translate the lab report well to the participant, so we are suggesting a more effective “translating material” used to explain the lab report to the patient.
Fourth, while the process of returning negative genomic screening results by mail was generally effective, our data suggest that mailed communications will not be optimal for all patients. Approximately one in five participants reported that they would have preferred that their results be returned in person. Many of these patients as well as individuals with demographic characteristics, such as less education or lower health literacy, which were associated with more difficulty in understanding the letter, might benefit from meeting with a genetic counselor or other appropriate medical specialist to discuss their results in greater detail. Additional research is needed to identify individuals who might benefit from a referral to a genetic counselor. Future screening activities might also benefit from the inclusion of alternative patient education and support strategies, such as group counseling sessions, online decision aids, or e-counseling tools.14
Additional research is also needed to determine if, and in what situations, mailed letters may not be appropriate for communicating certain kinds of negative genomic screening results. Previous data indicate that patients with a positive family history are more motivated to participate in genomic screening studies, which may lead these patients to interpret their screening results in a manner that is more consistent with diagnostic settings. For example, patients with a higher prior probability of disease due to a known family history may be more likely to interpret a negative genomic screening result as a negative diagnostic result, perhaps believing that the screening methods that were used had evaluated the specific genetic risk factor associated with their family history, which may not be an accurate assumption.24,25 Patients with a positive family history may also have difficulty integrating knowledge of a negative genomic screening result into ongoing disease management strategies and personal understandings of disease risk.26 These and other situational considerations highlight the potential need to communicate some negative genomic screening results in person. The impact a screening result has on one’s health is dependent on the severity of the condition(s) being assessed, the potential harms associated with misinterpreting the result, and the ancillary health beliefs that influence how that result is understood.
Study limitations
This study returned genomic screening results in a translational research study conducted at a single academic health center. We were unable to know whether participants actually read the mailed letter and laboratory report. Participants were older (mean age = 60.8 years) and predominantly white (96.5%). As a result, the findings we report may not be typical of patients receiving care elsewhere. More work examining return of negative results in minorities and participants with lower education levels is needed. We were not able to explore the reasons behind participant responses since data were collected via a mailed survey. Moreover, we were not able to clarify the reasons that participants had a high level of value but a limited understanding of results. Finally, there was a significant delay between the time patients were recruited for the genomic screening study and their receipt of individual screening results. This delay may have affected patient recall of their reasons for pursuing genomic screening as well as their opinions regarding the overall value of the results they received.
Our study also was not able to compare the reporting of negative screening results by mail to other communication approaches, such as in-person reporting of screening results by a health-care professional. In addition, our study returned results by mail using a single version of a results letter and did not compare alternative methods of communicating negative results in writing, which might have included different versions of the results letter or supplemental materials, such a frequently asked questions document. Our study also did not compare mailed communication to electronic communication of genomic screening results. Finally, were not able to assess how patients act based on the negative genomic screening results they receive. This is an important area for future research as it is unclear how the receipt of negative genomic screening impacts actual health behaviors.
Conclusion
Our data show that most patients value receiving negative genomic screening results and are comfortable receiving such results by mail. However, given the number of participants who reported difficulty understanding some aspect of their genomic screening results or were left with questions, it should not be assumed that mailing screening results to patients is sufficient to meet their needs. It is critical that future plans to implement genomic screening include resources for in-person genetic counseling to support those patients who would benefit from receiving their results in person. Given the large numbers of patients who will be offered some form of genomic screening in the future, future research should seek to clarify best practices for communicating negative genomic screening results and determine what ancillary educational materials patients find helpful.
References
Murray MF, Evans JP, Angrist M, et al. A proposed approach for implementing genomics-based screening programs for healthy adults. NAM Perspectives. March 2018. Washington, DC: National Academy of Medicine.
Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genet Med. 2011;13:499–504.
Skinner D, Raspberry KA, King M. The nuanced negative: meanings of a negative diagnostic result in clinical exome sequencing. Sociol Health Illn. 2016;38:1303–1317.
Condit C. Public understandings of genetics and health. Clin Genet. 2010;77:1–9.
Schrijver I, Aziz N, Farkas DH, et al. Opportunities and challenges associated with clinical diagnostic genome sequencing. J Mol Diagn. 2012;14:525–540.
Graves KD, Sinicrope PS, Esplen MJ, et al. Communication of genetic test results to family and health-care providers following disclosure of research results. Genet Med. 2014;16:294–301.
Graves KD, Wenzel L, Schwartz MD, et al. Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2010;19:648–654.
Bradbury A, Patrick-Miller L, Fetzer D, et al. Genetic counselor opinions of, and experiences with telephone communication of BRCA1/2 test results. Clin Genet. 2010;79:125–131.
Tabor HK, Stock J, Brazg T, et al. Informed consent for whole genome sequencing: A qualitative analysis of participant expectations and perceptions of risks, benefits, and harms. Am J Med Genet A. 2012;158A:1310–1319.
Kauffman TL, Wilfond BS, Jarvik GP, et al. Design of a randomized controlled trial for genomic carrier screening in healthy patients seeking preconception genetic testing. Contemp Clin Trials. 2017;53:100–105.
Tabor HK, Jamal SM, Yu J-H, et al. My46: a web-based tool for self-guided management of genomic test results in research and clinical settings. Genet Med. 2016;19:467–475.
Schmidlen TJ, Wawak L, Kasper R, García-España JF, Christman MF, Gordon ES. Personalized genomic results: analysis of informational needs. J Genet Couns. 2014;23:578–587.
Kullo IJ, Olson J, Fan X, et al. The Return of Actionable Variants Empirical (RAVE) Study, a Mayo Clinic Genomic Medicine Implementation Study: design and initial results. Mayo Clin Proc. 2018;93:1600–1610.
Sutton EJ, Kullo IJ, Sharp RR. Making pretest genomic counseling optional: lessons from the RAVE study. Genet Med. 2018;20:1157–1158.
Pacyna JE, Breitkopf CR, Jenkins SM, et al. Should pretest genetic counselling be required for patients pursuing genomic sequencing? Results from a survey of participants in a large genomic implementation study. J Med Genet. 2018;56:317–324.
Kaphingst K, Facio F, Cheng M-R, et al. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82:408–415.
Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med. 2008;5:e91
Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. American J Med Genet C Semin Med Genet. 2018;178:24–37.
Sukenik-Halevy R, Ludman MD, Ben-Shachar S, Raas-Rothschild A. The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing. Genet Med. 2015;18:372–377.
Christensen K, Dukhovny D, Siebert U, Green R. Assessing the costs and cost-effectiveness of genomic sequencing. J Pers Med. 2015;5:470–486.
Foley SB, Rios JJ, Mgbemena VE, et al. Use of whole genome sequencing for diagnosis and discovery in the cancer genetics clinic. EBioMedicine. 2015;2:74–81.
Sboner A, Mu X, Greenbaum D, Auerbach RK, Gerstein MB. The real cost of sequencing: higher than you think! Genome Biology. 2011;12:125.
Sung S, Forman-Hoffman V, Wilson MC, Cram P. Direct reporting of laboratory test results to patients by mail to enhance patient safety. J Gen Intern Med. 2006;21:1075–1078.
Haga SB, Mills R, Pollak KI, et al. Developing patient-friendly genetic and genomic test reports: formats to promote patient engagement and understanding. Genome Med. 2014;6:58.
Chen B. Good laboratory practices for molecular genetics testing. Crit Values. 2009;2:27–28.
Atkinson P, Featherstone K, Gregory M. Kinscapes, timescapes and genescapes: families living with genetic risk. Sociol Health Illn. 2013;35:1227–1241.
Acknowledgements
This study was supported by a grant from the US National Institutes of Health (U01 HG006379) and by the Mayo Clinic Center for Individualized Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stuttgen, K., Pacyna, J., Beck, A. et al. Patient reactions to receiving negative genomic screening results by mail. Genet Med 22, 1994–2002 (2020). https://doi.org/10.1038/s41436-020-0906-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0906-2