Abstract
Purpose
A COVID-19 pandemic business continuity plan (BCP) was rapidly developed to protect the Victorian newborn screening (NBS) program. Here, we present the outcomes of our COVID-19 BCP and its impact on the Victorian NBS laboratory service.
Methods
Change management principles were used to develop a BCP that included mapping of NBS processes against staff resources, triaging priorities, technology solutions, supply chain continuity, gap analysis, and supporting maternity service providers. The effect was assessed quantitatively by review of key performance indicator data and qualitatively from staff feedback.
Results
A four-stage BCP was implemented. Stage 1 split teams into two, which rotated weekly, onsite (laboratory) and offsite (home). At 20 weeks post-implementation the BCP only progressed to stage 1 and the overall turnaround time was maintained. Staff experience indicated benefits from the review of workflow but noted some social impact associated with the change.
Conclusion
The preparedness and agility of implementation was based on our focus on the newborn babies and their families, our production system, and a continuous improvement mindset. Both our people and technology infrastructure processes are crucial to this success as we continue to adapt to new challenges.
Similar content being viewed by others
INTRODUCTION
Newborn bloodspot screening (NBS) programs worldwide aim to test all neonates within their jurisdiction to prevent life threatening or life limiting consequences of disorders that can present soon after birth.1 The NBS program for the State of Victoria, Australia (representing 227,416 km2 and a population of 6.49 million2) is located within the Royal Children’s Hospital Melbourne campus. This laboratory is one of five in Australia and services 90 maternity service providers, testing approximately 80,000 newborns annually for 25 conditions, with an estimated overall disease incidence of 1 in 1,000 babies.3
The NBS laboratory, like diagnostic laboratories, is a production environment (generating around 12,000 test results per day) and the concepts of customer focus, workflow, quality, and continuous improvement are relevant. In 2019, the laboratory undertook significant process-driven change projects as part of our overall NBS efficiency drive. In the first part of this process the concepts of daily startup meetings, kaizen for small continuous improvements, kanban boards, and plan–do–check (study)–act cycle were implemented.4,5,6 In the second half of the year a formal efficiency project was conducted in conjunction with Toyota Production Systems Support Centre (TSSC) Australia and the staff were trained in a number of TSSC tools to visualize processes and status.7 As a result of these initiatives the NBS staff became more adaptive to change.
Change management is not simply about the technical process of project implementation, but importantly involves the management of staff expectations. At a high level, the three phases of change include the initial excitement and anxiety for the change, then the trough of disillusionment, and finally cautious optimism, with individual staff moving along this curve at different rates. The eventual change will involve the formation of a new habit or habits, which has a suggested median time of 10 weeks to establish.8 Once a team settles into a new habit, there can again be a reluctance to change. Hence, in the concept of improvement it becomes essential to maintain change as the norm to avoid stagnation and resistance. This is where the kaizen approach to continuous improvement aids the agility of change.5 However, it is important to pace and monitor change to ensure it is effectively implemented and does not cause unintended disruption.
Disruption of a single step in the screening pathway could have serious consequences for the newborn. On 11 March 2020, the potential for disruption to NBS services worldwide was realized, when the spread of the novel coronavirus, SARS-CoV-2, was declared a global pandemic by the World Health Organization (WHO). This was less than 12 weeks after the “pneumonia of unknown cause” from Wuhan, China was reported to the WHO.9 A mere five days later (on 16 March 2020), the NBS laboratory in the State of Victoria, Australia commenced the implementation of a rapidly developed COVID-19 pandemic business continuity plan (BCP). While contingency planning had always been in place as part of ISO15189 accreditation, the scale of potential disruption seen with COVID-19 had previously never been considered.
Here, we present the outcomes of the first 20 weeks of our COVID-19 BCP and the impact, both positive and negative, these changes have had on the Victorian NBS laboratory service.
MATERIALS AND METHODS
NBS laboratory setup
The total testing pathway for screening commences with the birth of the baby, and is followed by dried bloodspot (DBS) sample collection by the maternity service provider (aiming for 48–72 hours of age for first collection), transport of the sample to the laboratory, testing of the DBS sample, reporting of the normal results (screen negative), a request for a repeat sample when the sample is inadequate or ambiguous results are obtained, or referral of abnormal (screen positive) results to the appropriate clinical specialist service.
The laboratory is staffed by two clinical scientists (Head and Deputy Head), three grade 2 medical scientists, four grade 1 medical scientists, a technician, a newborn screening nurse educator, and an administrative assistant. These 12 staff members make up the overall full time equivalent profile of 9.6 staff. The laboratory equipment is integrated into the laboratory information management system (LIMS). An overview of the laboratory, including video, can be found at https://www.vcgs.org.au/tests/newborn-bloodspot-screening.
COVID-19 BCP development process
The objective of our BCP was to provide a framework to ensure effective response and minimize risk to the Victorian NBS service during the COVID crisis. The impetus for the plan was identification of the NBS service as a critical operation. The process commenced with a request from the Victorian Clinical Genetics Services (VCGS) Executive, following advice from the State Government, to develop a BCP to cover the next six months of operation. The plan required splitting the workforce into two teams commencing in ten days’ time. As there was no previous plan developed, the BCP was developed from first principles using the following ten steps:
-
1.
Develop initial high level BCP. Considerations for the plan included identification of triggers to move between stages, what action(s) needed to be undertaken upon identification of the trigger, hurdles associated with moving to the next stage, limitations for the NBS program during this stage, and immediate actions to be proactively taken in preparation for each stage.
-
2.
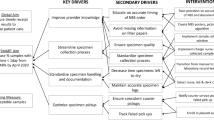
Map NBS processes against staff resources. A visual tool developed in association with TSSC was used to map processes against staff. The tool uses a magnetic poster board that has a grid setup with time of day on one axis and staff member on the other access. Preprepared magnets (detailing components of the NBS process) were placed on the board and then reordered based on team discussion to visualize workflow. The NBS manager facilitated the discussion.
-
3.
Split NBS into two teams. Individuals were initially paired based on skill sets with codependences identified as administration support, mass spectrometry technical support, immunoassay technical support, validation of results, and team management. Then, all staff worked together to optimize the teams. The process here was management facilitated discussions with staff physically present in a large common space and photos of staff that could not be present. Staff would then reorganize into groups physically across two areas of the room, which would continue until a consensus was reached.
-
4.
Review priorities. Triage work into essential activities, important activities, and nonessential activities. Decide on what projects to bring forward to support the NBS service during the COVID-19 crisis and what projects have lower priority.
-
5.
Identify gaps. Look at gaps in the new proposed operation that need to be urgently addressed.
-
6.
Audit consumables and supply chain to identify issues and ensure six-month continuous supply.
-
7.
Support maternity service providers. Monitor feedback and concerns raised by maternity providers in Excel and categorize into groups. Respond with group communications that address frequently asked questions and to individuals as concerns are raised.
-
8.
Train non-NBS staff. Look for opportunities to do rapid preliminary familiarization training of non-NBS VCGS scientific staff.
-
9.
Review standard operation procedures to ensure they are sufficiently clear and up-to-date for non-NBS scientific support and training needed.
-
10.
Continue to review compliance of BCP and workforce as Government and workplace regulations are updated.
Quantitative outcome analysis
To determine if there was a significant change in the NBS service delivery as a consequence of the COVID-19 associated restrictions, we looked at the three key performance indicators (KPIs) routinely reported to the Victorian Department of Health and Human Services.3 These quantitative indicators were examined for each of the four full months from April to July 2020 and were compared with the same period in the previous two years:
-
1.
Timeliness of sample collection. The definition for this KPI is the percentage of DBS cards collected within the agreed standard timeframe, with the denominator being the total number of first sample newborn screening cards received by the laboratory. The benchmark for the performance of this indicator is that 95% of babies born in Victoria should have a sample collected before 72 hours of age.1
-
2.
Timeliness of sample transport. The definition for this KPI is the percentage of DBS cards received in the laboratory within the agreed standard timeframe, with the denominator being the total number of first sample newborn screening cards received by the laboratory. The benchmark for the performance of this indicator is that 95% of samples should be in transit for less than 96 hours.10
-
3.
Timeliness of screening and reporting of results to all hospitals/providers. The definition for this KPI is the percentage of newborn bloodspot results reported to the maternity provider within the agreed standard timeframe, with the denominator being the total number of first sample newborn screening cards received by the laboratory. The benchmark for the performance of this indicator is that 95% of babies born in Victoria should have an NBS result by 9 days of age.
All data for the quantitative analysis were downloaded from the NBS LIMS system and then further summarized using Microsoft Excel 2013 pivot tables and graphs. Analysis of variance (ANOVA) was performed with p < 0.05 considered to demonstrate a statistically significant difference.
Qualitative outcome analysis
Qualitative analysis of the NBS Laboratory COVID-19 response involved the following:
-
1.
Group discussion with the NBS teams. At 20 weeks, each member of the NBS was asked to list a positive and a negative impact to the implementation of the BCP and COVID-related changes.
-
2.
Monitoring of referral process for screen positive babies. In general, the referral process involves contacting the designated (based on the baby’s post code) medical support service, either at the Royal Children’s Hospital or Monash Children’s Health. The baby’s parents are then contacted and arrangements made for follow-up tests and consultation.
RESULTS
Timeline
A four-stage BCP was developed and implemented in a 12-day period (8 working days) for the Victorian NBS program (Table 1). The prerequisite for the BCP was the need to split the current NBS team into two workforces to contain any COVID-19 infections to one team in the event of a staff member testing positive for the virus; this split was a common response to the COVID-19 crisis nationally and internationally.11,12
The realization that the acute situation was turning chronic with the start of the second wave led to the reassessment of the COVID plan and the strength of the teams for longevity. Additional actions undertaken were the review and revision of a critical onsite staff list and 24/7 support from our Human Resources team to enable rapid issue of permitted worker permits and permits to allow staff continued access to childcare. These safeguarded continuation of onsite and offsite work.
The outcomes represent the first 20 weeks of working in the two team model. The Victorian NBS laboratory demonstrated its successful adaptation to the “new normal” of two rotating teams (A and B), with two staff members working from home for the duration, as they did not meet the COVID-safe criteria to be allowed onsite. No staff tested positive for COVID-19 at the 20-week review period and consequently the BCP only escalated to stage 1.
The timeline for these key events is shown in Fig. 1.
The business9,24 continuity plan (BCP) and splitting of the team occurred in March 2020. By 10 weeks the split teams had settled into their new routine and the first wave (April–May 2020) associated with international travelers had passed. The risk of infection increased with the second wave (July–November 2020) associated with community transmission. This second wave was specific to Victoria, with other states of Australia and New Zealand moving back to one team by mid-2020. WHO World Health Organization.
Development of split workload and IT enablers
A variety of information technology (IT) solutions were quickly deployed to support the home workforce. These can be broadly divided into two groups: (1) assisting productivity, which included purchase of laptop computers for each staff member and/or configuration of personal laptops/computers, assignment of virtual private network (VPN) access, upgrade of the VPN server; and (2) communication and collaboration, which included sign-up to video conference and instant messaging applications. This included the continuation of our daily short-form morning work planning meetings, but now changed to video conference. In addition, instant messaging proved useful for quick communications between onsite and offsite teams, supplemented as needed by telephone and email communication.
The work was generally divided into what could and what could not be performed from home. Tasks were generally divided as shown in Table 2. An example early challenge, which resulted in an IT solution, was data entry from home where tracking which DBS cards needed registration was difficult. In our previous model, staff would data enter the cards physically in front of them so it was easy to keep track of what work was done and/or required. Moving to relying on electronic scans of the DBS cards meant a new process of allocating blocks of DBS cards to be data entered to staff members was required. The staff initially communicated this manually via instant messaging, but it soon became clear that a solution using the information management system was required. A solution was put in place, which allowed the staff to track all DBS cards required for data entry, assign a block of them to a staff member, and then loop through the assigned DBS cards to perform data entry. As each DBS card sample was opened for data entry, the associated scan would open at the same time. This saved significant staff time and streamlined the process for staff onsite and offsite.
Maternity service providers
Maternity service providers in many jurisdictions, including ours, faced significant challenges in performing the normal NBS routine due to the COVID-19 outbreak. Questions arose about the infection risk of NBS cards13 and early discharge of mothers. In some instances, mothers were being discharged home early, often within 24 hours of delivery, and sometimes midwives were not allowed to enter the home because of the fear of COVID-19. In cases where the mother was discharged early (whether self or hospital initiated), we perceived a significant risk that a newborn screening test may be missed. In late March 2020 we put out a communication to all hospitals offering support and advising to collect a sample before discharge. This communication caused significant confusion leading to a high level of email and phone traffic at a time when the two teams were still adjusting. In hindsight the communication, while well intended and reviewed by our internal communications team before release, was less than ideal. A follow-up communication was required to help clarify the message. This serves as the main example of a less than ideal quick decision made at the start of the COVID-19 pandemic. Fortunately, the other decisions made to support maternity service providers demonstrated a significant improvement in processes.
Projects to reduce the burden on maternity service providers were reprioritized due to the COVID-19 pandemic. Two projects were brought forward and rapidly implemented in two weeks after splitting the teams.
-
1.
Sample collection timeframe. The rapid review of NBS data from babies who were sampled earlier than our generally recommended 48–72 hour window showed there were only minor changes in the distribution of analyte levels for samples collected at 24–47 hours. This was confirmed by data provided by two other Australasian (New Zealand and Western Australia) NBS laboratories. We took a more conservative approach and decided to accept samples collected at 36–47 hours, previously considered too early, to reduce the need for re-collection in babies discharged in this time window.
-
2.
Review of protocol for unsuitable samples. The Human Genetics Society of Australasia guideline states that the target unsuitable sample rate is less than 0.5%.10 The Victorian NBS Laboratory in recent years had developed stringent criteria for suitability of a DBS, resulting in percentage of unsuitable samples requiring re-collection reaching 2%. This meant that the laboratory’s requests for re-collections were high compared with the guideline and also to other Australian NBS services. While this was problematic previously, the advent of COVID-19 increased the urgency to find a resolution to this issue. An extra vetting step was established for a higher-level review by the Head or Deputy Head of the NBS service for the suitability of a sample and the need for re-collection. We have met the KPI for unsuitable samples for May, June, and July 2020 (<0.5% rejection rate). Important outcomes were (1) simplification of process and paperwork; (2) fewer re-collections and invasive procedures for the baby, while maintaining quality; and (3) meeting the national guideline and matching other states.
Quantitative and qualitative outcome analysis
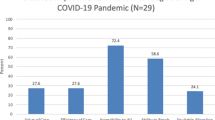
The frequency of review of the laboratory’s three turnaround time KPIs increased from quarterly to at least monthly as part of the change process. Quantitative analysis demonstrates that we have continued to meet the 95% criteria for collection within 72 hours, delivery within four days (with the exception of April and June), and results within nine days of life during the first four months of the COVID-19 BCP. The ANOVA analysis of the aggregated data for April–July 2018, 2019, and 2020 demonstrated a statistically significant improvement in the collection of samples within 72 hours of life. There was no difference seen to the parameters for delivery of samples within 4 days or results within 9 days of life (Fig. 2).
The indicators are (a) collected within 72 hours of birth, (b) delivery within 4 days of collection, and (c) results within 9 days of life. Acceptable performance for each indicator is 95% or greater. There was no statistical change in these indicators due to COVID. Analysis of variance (ANOVA) for collected within 72 hours of birth (p = 0.0001, with an overall improvement seen during COVID), delivery within four days of collection (p = 0.59), and final results by nine days of age (p = 0.86).
There were 70 referrals (representing 1 in 366 babies) for the four-month period, related to screen positive results associated with congenital hypothyroidism (n = 20), cystic fibrosis (n = 38), and metabolic conditions (n = 12). The referral process remained unchanged during the COVID-19 restrictions. One of these 70 referrals, a screen positive result for low free carnitine, was noted to be problematic, with a significant delay of two weeks before a sample was sent for follow-up testing to the VCGS Metabolic Laboratory. The baby was otherwise considered healthy based on local medical assessment. This baby lived approximately 1 hour outside of a regional center, located 5 hours’ drive to Melbourne. The major obstacle was the logistics of sample transport with the suspension of flights.
Qualitative reactions of staff to the COVID-19 changes was sought during a group meeting in the middle of June 2020, where all team members were asked to provide a positive and a negative aspect of the changes. Overall, the meeting and staff general comments were positive with respect to the new ways of working; however, social aspects were highlighted as the primary negative components of the changes (Table 3).
DISCUSSION
In this study, we have outlined the development, deployment, and outcomes associated with our BCP. This plan allowed us to successfully continue NBS operations while adhering to government and institutional restrictions during the COVID-19 pandemic. We only needed to move to stage 1 and prepare for stage 2. Stages 3 and 4 did not need to be implemented. In reality, stage 3 (sending the samples interstate) was not realistic during the first wave due to the concurrent burden that COVID-19 placed on all NBS services nationally but was theoretically possible during the second wave. This meant that, depending on coincident COVID-19 transmission rates in other states, the BCP may have been reduced to effectively three workable stages, 1, 2, and 4, bypassing stage 3. Overall, the two team model, rotating weekly between home and onsite, was very successful and staff adjusted relatively easily to this change.
Our BCP focused primarily on factors internal to the NBS laboratory but rapid responses to external factors were also required. An example was the decision to modify our collection guidelines and accept samples collected earlier (36–72 hours) than previously recommended (48–72 hours). This was a response to the earlier discharge of infants during the pandemic. We were aided in making this decision rapidly by data kindly provided by other Australian NBS programs.
The agility of the development and implementation of the BCP was counterbalanced by the time taken to involve all stakeholders. All staff working were involved in the decision making processes, but there was insufficient time to wait for the input of staff who were on leave or were not working on a particular day.14 Managing our newly remote teams and establishment of the new routines of communication and supporting trust in remote work were important to the overall success of the BCP.15 Overall, our ability to successfully adapt as demonstrated by our quantitative and qualitative outcomes is drawn from the development of Toyota kaizen processes for continuous improvement, increasing the adaptive mindset of staff.
There are two components of the small continuous improvement process associated with kaizen; they are change and sustain. The change component can have a number of negatives that require management, which include miscommunication, people disagree with the change, errors, variation, and the unforeseen.5 The sustain component looks at standardization and normalization of the process. Managing these change and sustain components requires extra work and the plan–do–check (study)–act cycle is fundamental in its success.4,16 The development of a BCP for medical laboratories is complicated and while the hastiness of our numerous, multifaceted COVID-19 related change decisions demonstrated agility and teamwork, they also brought risks of not considering all the consequences of decisions made. Hence, this illustrated the importance of studying the data that demonstrated the success of a change and quickly acting on problems to ensure overall success and sustainability. Conversely, failing to adequately monitor change can contribute to disillusionment in management decision processes and undermines the effectiveness of this change and staff engagement in future changes.
All staff members are committed to the quality of the Victorian NBS operation and see quality as the way forward. As this pandemic has occurred in the age of the fourth industrial revolution, a number of elements of BCP stage 1 included the rapid uptake and embracing of new and disruptive technologies,17,18 to assist remote working and communication in a virtual environment. However, in preparing the BCP it was the visual and sometimes simple tools, such as the TSSC magnetic board used to develop the new roster and workflow, that supported the communication and engagement of staff in the rapid change process. This also was a project planned for the future but brought forward due to the reprioritization of tasks related to the COVID-19 pandemic.
Moving to the future, we will work on further improving our data entry process by leveraging technology available to us. The current scanning software for our DBS cards has built-in intelligent character recognition (ICR) and optical character recognition (OCR) technology that could be used to automatically read the DBS card information from the hospital Bradmar labels and import the data directly into the laboratory information management system.19 This would reduce the time and improve quality for the data entry process. We will also work with major hospitals to interface with their hospital systems (including electronic medical records) where possible to streamline the data entry and reporting processes. Furthermore, for our IT infrastructure, we are moving toward using a virtualized application server allowing software to run on shared infrastructure with improved security, higher performance, and that is more scalable than remote virtual machine access without the need for VPN access. This will give our staff increased flexibility to access the laboratory information management system and complete their work.
Overall, this pandemic has been and continues to be an experimental exercise in rapid adaptation. The original BCP was a reaction to the acute situation of increase in COVID-19 in our community and the application of the COVID-19 change process and decisions highlighted here can be applied broadly. The use of data driven insights demonstrate the success to date of our plan. The reasons for this success are multifaceted and include the relatively low burden of COVID-19 disease in Australia compared with other countries,20,21,22,23 agility to change significantly helped by the TPS project in 2019, and the capability and dedication of each member of staff who together constituted the two teams. Finally, the NBS-specific needs were underpinned by organizational adherence to good laboratory practice and overarching clinical governance principles.
Conclusions
In this study, we have presented the overall successful implementation of the business continuity plan for the Victorian NBS laboratory service during the COVID-19 pandemic. This allowed us to maintain the service with no discernible effects on its quality despite the challenges of rapidly adapting to new work processes and working within government restrictions. The preparedness and agility of implementation was based on our focus on the newborn babies and families, our production system, and a continuous improvement mindset. Both our people and IT infrastructure processes are crucial to this current and future success as we continue to face new challenges.
Data availability
There are no specific genetic data presented in this paper. The summary data presented to underpin the quantitative outcomes are generated from the laboratory database.
References
Department of Health, Commonwealth of Australia. Newborn bloodspot screening national policy framework. p. 76 (Canberra, Australia, 2017).
Population Australia 2020. Population of Victoria. http://www.population.net.au/population-of-victoria/ (2020).
Victorian Government Department of Health and Human Services, Prevention and Population Health Branch. Newborn bloodspot screening policy and guidelines. p. 9 (Melbourne, Victoria, Australia, 2018).
Moen, R. D. & Norman, C. L. Circling back: clearing up myths about the Deming cycle and seeing how it keeps evolving. Quality Progress 2010. http://www.apiweb.org/circling-back.pdf. https://asq.org/quality-resources/pub/quality-progress (2010).
Greaves, R. F. Kaizen Continuous improvement. Vietnam Chemical Pathology Course. https://eacademy.ifcc.org/events/vietnam-chemical-pathology-courses/?ctype=1154&cid=1871 (2017).
Magee, D. How Toyota Became #1: Leadership Lessons from the World’s Greatest Car Company. (Portfolio, New York, USA, 2008).
Toyota Production System Support Centre (TSSC) Australia. https://tssc.com.au/ (2020).
Gardner, B., Lally, P. & Wardle, J. Making health habitual: the psychology of ‘habit-formation’ and general practice. Br. J. Gen. Pract. 62, 664–666 (2012).
World Health Organization (WHO). Novel corona-virus-19: events as they happened. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (2020).
Human Genetics Society of Australasia. Newborn bloodspot testing. https://www.hgsa.org.au/documents/item/29 (2011).
ISNS. Logistical problems due to corona virus. International Society of Neonatal Screening (ISNS), Members Forum. https://membership.isns-neoscreening.org/member/forum/21/ (2020).
NewSTEPs. COVID 19 NBS response continuity operations plans United States. Association of Public Health Laboratories. https://www.newsteps.org/index.php/resources/covid-19-nbs-response-continuity-operations-plans-coop (2020).
Chin, A. W. H. et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020 1, e102020 (2020).
Pulakos, E. & Kaiser, R. Collaboration: don’t let teamwork get in the way of agility. Harvard Business Review. https://hbr.org/2020/05/dont-let-teamwork-get-in-the-way-of-agility (2020).
Larson, B. Z., Vroman, S. R. & Makarius, E. E. Leading teams: a guide to managing your (newly) remote workers. Harvard Business Review. https://hbr.org/2020/03/a-guide-to-managing-your-newly-remote-workers (2020).
Deming, W. E. Plan do study act cycle. The W. Edwards Deming Institute. https://deming.org/explore/pdsa/ (2020).
Sung, H. H., Choi, K.-M., Jun, Y. H. & Cho, E. K. Study on the fourth industrial revolution and clinical laboratory science techniques. Korean J. Clin. Lab. Sci. 51, 386–395 (2019).
Greaves, R. F. et al. Key questions about the future of laboratory medicine in the next decade of the 21st century: a report from the IFCC-Emerging Technologies Division. Clin. Chim. Acta 495, 570–589 (2019).
Brynjolfsson, E. M. A. The business of artificial intelligence. Harvard Business Review. https://hbr.org/2017/07/the-business-of-artificial-intelligence (2017).
Statista. COVID-19 infection density countries most total cases 2020. https://www.statista.com/chart/21176/covid-19-infection-density-in-countries-most-total-cases/ (2020).
Statista. Australia coronavirus cases per 100,000 population by state 2020. https://www.statista.com/statistics/1103944/australia-coronavirus-cases-per-100-000-population-by-state/ (2020).
Statista. Novel corona virus 2019ncov cases worldwide by country 2020. https://www.statista.com/statistics/1043366/novel-coronavirus-2019ncov-cases-worldwide-by-country/ (2020).
Statista. Coronavirus deathy worldwide per million inhabitants 2020. https://www.statista.com/statistics/1104709/coronavirus-deaths-worldwide-per-million-inhabitants/ (2020).
Mclean, H. & Huf, B. Emergency powers, public health and COVID-19. Research Paper No. 2, August 2020. Department of Parliamentary Services, Parliament of Victoria. p. 70 (Melbourne, 2020).
Acknowledgements
We are grateful for the support of Biochemical Genetics Staff, VCGS, and Murdoch Children’s Research Institute (MCRI) Leadership Teams. Research conducted at the MCRI was supported by the Victorian Government’s Operational Infrastructure Support Program. We are also appreciative of the solidarity and support of our sister laboratories across Australia and New Zealand. Throughout the early stages of the pandemic, Australasian NBS laboratories supported each other via fortnightly teleconferences organized by the NBS National Program Management Committee. These meetings were helpful in providing updates on supply chain issues, NBS management strategies during the pandemic, and sharing data from samples collected earlier than the recommended screening times. The Victorian Newborn Bloodspot Screening Program is funded by the Department of Health and Human Services Victoria.
Author information
Authors and Affiliations
Contributions
All authors contributed to the change management process. J.C. conceptualized the idea for this paper. R.F.G. designed and developed the initial business continuity plan (with direction of the split team and timeline provided by Senior Management), which encompassed the formal analysis, methodology, and visualization. J.P. and M.W. refined, adapted and approved the B.C.P. R.F.G., J.P., and M.W. administered the project. J.C., J.P., and R.F.G. reviewed the clinical data associated with change decisions. C.M. initiated and supported the software integration that formed the technology solutions. R.F.G. wrote the first draft and coordinated the author input. All authors contributed to the writing and review of the manuscript and approved the final version for submission to GIM.
Corresponding author
Ethics declarations
Ethics Declaration
Ethics approval was not required as this work was a management audit of processes. We confirm this work did not involve research experiments on humans or animals. All processes described in this paper related to our day to day screening work as part of ISO15189 accreditation. The screening results were cumulated to determine the calculations for the key performance indicators related to turnaround time.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greaves, R.F., Pitt, J., McGregor, C. et al. Newborn bloodspot screening in the time of COVID-19. Genet Med 23, 1143–1150 (2021). https://doi.org/10.1038/s41436-020-01086-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-01086-6