Abstract
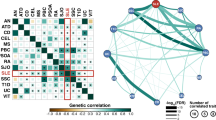
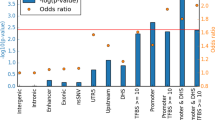
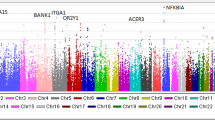
Primary antiphospholipid syndrome is characterized by thrombosis and autoantibodies directed against phospholipids or associated proteins. The genetic etiology of PAPS remains unknown. We enrolled 21 patients with thromboembolic events associated to lupus anticoagulant, anticardiolipin and anti β2 glycoprotein1 autoantibodies. We performed whole exome sequencing and a systematic variant-based analysis in genes associated with thrombosis, in candidate genes previously associated with APS or inborn errors of immunity. Data were compared to public databases and to a control cohort of 873 non-autoimmune patients. Variants were identified following a state-of-the-art pipeline. Enrichment analysis was performed by comparing with the control cohort. We found an absence of significant HLA bias and genetic heterogeneity in these patients, including when testing combinations of rare variants in genes encoding for proteins involved in thrombosis and of variants in genes linked with inborn errors of immunity. These results provide evidence of genetic heterogeneity in PAPS, even in a homogenous series of triple positive patients. At the individual scale, a combination of variants may participate to the breakdown of B cell tolerance and to the vessel damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Original data are available in Supplemental material.
Change history
19 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41435-024-00261-y
References
Hughes GR. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br Med J. 1983;287:1088–89.
Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med. 2018;379:1290.
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306.
Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118:4714–8. https://doi.org/10.1182/blood-2011-03-340232
Dieudonné Y, Guffroy A, Poindron V, Sprauel PS, Martin T, Korganow A-S, et al. B cells in primary antiphospholipid syndrome: Review and remaining challenges. Autoimmun Rev. 2021;20:102798.
Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7:330–9.
Moschetti L, Dal Pozzolo L, Le Guern V, Morel N, Yelnik CM, Lambert M, et al. Gender differences in primary antiphospholipid syndrome with vascular manifestations in 433 patients from four European centres. Clin Exp Rheumatol. 2022;40:19–26.
Cervera R. Antiphospholipid syndrome. Thromb Res. 2017;151:S43–S47.
Matthey F, Walshe K, Mackie IJ, Machin SJ. Familial occurrence of the antiphospholipid syndrome. J Clin Pathol. 1989;42:495–7.
Bansal AS, Hogan PG, Gibbs H, Frazer IH. Familial primary antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39:705–6.
Ravindran V, Rajendran S, Elias G. Primary antiphospholipid syndrome in monozygotic twins. Lupus. 2013;22:92–4.
Cevallos R, Darnige L, Arvieux J, Veyssier P, Gruel Y. Antiphospholipid and anti-beta 2 glycoprotein I antibodies in monozygotic twin sisters. J Rheumatol. 1994;21:1970–1.
Rodríguez-García ME, Cotrina-Vinagre FJ, Bellusci M, Martínez de Aragón A, Hernández-Sánchez L, Carnicero-Rodríguez P, et al. A novel de novo MTOR gain-of-function variant in a patient with Smith-Kingsmore syndrome and Antiphospholipid syndrome. Eur J Hum Genet. 2019;27:1369–78.
Dieudonné Y, Guffroy A, Vollmer O, Carapito R, Korganow A-S. IKZF1 Loss-of-Function Variant Causes Autoimmunity and Severe Familial Antiphospholipid Syndrome. J Clin Immunol. 2019;39:353–7.
Goldstein R, Moulds JM, Smith CD, Sengar DP. MHC studies of the primary antiphospholipid antibody syndrome and of antiphospholipid antibodies in systemic lupus erythematosus. J Rheumatol. 1996;23:1173–9.
Ortiz-Fernández L, Sawalha AH. Genetics of antiphospholipid syndrome. Curr Rheumatol Rep. 2019;21:65.
Caliz R, Atsumi T, Kondeatis E, Amengual O, Khamashta MA, Vaughan RW, et al. HLA class II gene polymorphisms in antiphospholipid syndrome: haplotype analysis in 83 Caucasoid patients. Rheumatology. 2001;40:31–6.
Domenico Sebastiani G, Minisola G, Galeazzi M. HLA class II alleles and genetic predisposition to the antiphospholipid syndrome. Autoimmun Rev. 2003;2:387–94.
Asherson RA, Doherty DG, Vergani D, Khamashta MA, Hughes GR. Major histocompatibility complex associations with primary antiphospholipid syndrome. Arthritis Rheum. 1992;35:124–5.
Prieto GA, Cabral AR, Zapata-Zuñiga M, Simón AJ, Villa AR, Alarcón-Segovia D, et al. Valine/valine genotype at position 247 of the beta2-glycoprotein I gene in Mexican patients with primary antiphospholipid syndrome: association with anti-beta2-glycoprotein I antibodies. Arthritis Rheum. 2003;48:471–4.
Swadzba J, Sanak M, Iwaniec T, Dziedzina S, Musiał J. Valine/Leucine247 polymorphism of beta2-glycoprotein I in patients with antiphospholipid syndrome: lack of association with anti-beta2-glycoprotein I antibodies. Lupus. 2006;15:218–22.
Yasuda S, Atsumi T, Matsuura E, Kaihara K, Yamamoto D, Ichikawa K, et al. Significance of valine/leucine247 polymorphism of?2-glycoprotein I in antiphospholipid syndrome: Increased reactivity of anti-?2-glycoprotein I autoantibodies to the valine247?2-glycoprotein I variant. Arthritis Rheum. 2005;52:212–8.
Chaturvedi S, Braunstein EM, Yuan X, Yu J, Alexander A, Chen H, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135:239–51.
Chaturvedi S, Brodsky RA, McCrae KR. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front Immunol. 2019;10:449.
Barinotti A, Radin M, Cecchi I, Foddai SG, Rubini E, Roccatello D, et al. Genetic Factors in Antiphospholipid Syndrome: Preliminary Experience with Whole Exome Sequencing. IJMS. 2020;21:9551.
Liang Y-L, Wu H, Shen X, Li P-Q, Yang X-Q, Liang L, et al. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol Biol Rep. 2012;39:8873–82.
Yin H, Borghi MO, Delgado-Vega AM, Tincani A, Meroni P-L. Alarcón-Riquelme, Association of STAT4 and BLK, but not BANK1 or IRF5, with primary antiphospholipid syndrome. Arthritis Rheum. 2009;60:2468–71.
Islam A, Khandker SS, Alam F, Kamal MA, Gan SH. Genetic risk factors in thrombotic primary antiphospholipid syndrome: A systematic review with bioinformatic analyses. Autoimmun Rev. 2018;17:226–43.
Iuliano A, Galeazzi M, Sebastiani GD. Antiphospholipid syndrome’s genetic and epigenetic aspects. Autoimmun Rev. 2019;18:102352.
Zhang P, Philippot Q, Ren W, Lei W-T, Li J, Stenson PD, et al. Genome-wide detection of human variants that disrupt intronic branchpoints. Proc Natl Acad Sci USA. 2022;119:e2211194119.
Bigio B, Seeleuthner Y, Kerner G, Migaud M, Rosain J, Boisson B, et al. Detection of homozygous and hemizygous complete or partial exon deletions by whole-exome sequencing. NAR Genomics Bioinforma. 2021;3:lqab037.
Mbatchou J, Barnard L, Backman J, Marcketta A, Kosmicki JA, Ziyatdinov A, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53:1097–103.
Simone B, De Stefano V, Leoncini E, Zacho J, Martinelli I, Emmerich J, et al. Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate reductase C677T: a meta-analysis involving over 11,000 cases and 21,000 controls. Eur J Epidemiol. 2013;28:621–47.
Eppenberger D, Nilius H, Anagnostelis B, Huber CA, Nagler M. Current Knowledge on Factor V Leiden Mutation as a Risk Factor for Recurrent Venous Thromboembolism: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022;9:883986.
Chiasakul T, De Jesus E, Tong J, Chen Y, Crowther M, Garcia D, et al. Inherited Thrombophilia and the Risk of Arterial Ischemic Stroke: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e012877.
Gragert L, Madbouly A, Freeman J, Maiers M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum Immunol. 2013;74:1313–20.
Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2022;42:1473–1507.
Yonal I, Hindilerden F, Hancer VS, Artim-Esen B, Daglar A, Akadam B, et al. The impact of platelet membrane glycoprotein Ib alpha and Ia/IIa polymorphisms on the risk of thrombosis in the antiphospholipid syndrome. Thromb Res. 2012;129:486–91.
Ochoa E, Iriondo M, Manzano C, Fullaondo A, Villar I, Ruiz-Irastorza G, et al. LDLR and PCSK9 Are Associated with the Presence of Antiphospholipid Antibodies and the Development of Thrombosis in aPLA Carriers. PLoS One. 2016;11:e0146990.
Rand JH, Wu X-X, Andree HAM, Lockwood CJ, Guller S, Scher J, et al. Pregnancy Loss in the Antiphospholipid-Antibody Syndrome — A Possible Thrombogenic Mechanism. N Engl J Med. 1997;337:154–60.
Al-Mubarak B, Abouelhoda M, Omar A, AlDhalaan H, Aldosari M, Nester M, et al. Whole exome sequencing reveals inherited and de novo variants in autism spectrum disorder: a trio study from Saudi families. Sci Rep. 2017;7:5679.
Alves M, Gomez-Villafuertes R, Delanty N, Farrell MA, O’Brien DF, Miras-Portugal MT, et al. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia. 2017;58:1603–14.
Neto NSR, Strunz CC, de Carvalho JF. Prevalence of genetic thrombophilia in primary antiphospholipid syndrome. Eur Rev Med Pharmacol Sci. 2021;25:3645–6.
Ames PR, Margaglione M, Tommasino C, Bossone A, Iannaccone L, Brancaccio V. Impact of plasma homocysteine and prothrombin G20210 A on primary antiphospholipid syndrome. Blood Coagul Fibrinolysis. 2001;12:699–704.
Green PHR, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43.
van Zeben D, Hazes JM, Zwinderman AH, Cats A, Schreuder GM, D’Amaro J, et al. Association of HLA-DR4 with a more progressive disease course in patients with rheumatoid arthritis. Results of a followup study. Arthritis Rheum. 1991;34:822–30.
Eikelboom JW, Weitz JI. The mTORC Pathway in the Antiphospholipid Syndrome. N Engl J Med. 2014;371:369–71.
Acknowledgements
The authors sincerely thank the patients for participating in this study. We thank Pr. Laurent Abel (HLA analysis) and Dr Isabelle Andre (Moesin variation) for fruitful discussions. We thank the patients and their families for participating in our study.
Funding
This work was supported by Strasbourg Hospital and University, by the France’s National Research Agency (Agence Nationale de Recherche; ANR), the Investment for the Future Program (Programme des Investissements d’Avenir; PIA) through a “Laboratoire d’Excellence” (LabEx) TRANSPLANTEX [ANR-11-LABX-0070_TRANSPLANTEX] as well as by Strasbourg’s Interdisciplinary Thematic Institute (ITI) for Precision Medicine, TRANSPLANTEX NG, as part of the ITI 2021-2028 program of the University of Strasbourg, CNRS and INSERM, funded by IdEx Unistra [ANR-10-IDEX-0002] and SFRI-STRAT’US [ANR-20-SFRI-0012]. Additional funding was provided by INSERM (UMR_S 1109), the Fédération Hospitalo-Universitaire (FHU) OMICARE, MSD Avenir “Autogen”, and finally the European regional development fund (European Union) INTERREG IV (project RARENET) and V programs (project PERSONALIS).
Author information
Authors and Affiliations
Contributions
Conceptualization AG, ASK, VG, RC, BB, AC, JLC Methodology AG, ASK, RC, BB, AC, JLC Investigation AG, LJ, YS, NP, VP, FM, VD, ACV, PZ, BN, AM, MJA, ASK, RC, AC, BB Data Curation ASK, AG, AC, BB, LJ, VD Writing, Review & Editing all authors, Funding Acquisition ASK,TM, RV, PSP, RC, SB, JLC, AC.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Commission Nationale Informatique et Liberté (CNIL) and the Institutional Board (CPP13/48). All the enrolled subjects provided written informed consent and the study was performed according to the principles of the Helsinski declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guffroy, A., Jacquel, L., Seeleuthner, Y. et al. An immunogenomic exome landscape of triple positive primary antiphospholipid patients. Genes Immun 25, 108–116 (2024). https://doi.org/10.1038/s41435-024-00255-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-024-00255-w