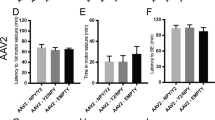
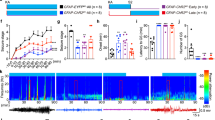
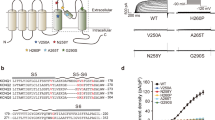
Abstract
Gene therapy offers a potential alternative to the surgical treatment of epilepsy, which affects millions of people and is pharmacoresistant in ~30% of cases. Aimed at reducing the excitability of principal neurons, the engineered expression of K+ channels has been proposed as a treatment due to the outstanding ability of K+ channels to hyperpolarize neurons. However, the effects of K+ channel overexpression on cell physiology remain to be investigated. Here we report an adeno-associated virus (AAV) vector designed to reduce epileptiform activity specifically in excitatory pyramidal neurons by expressing the human Ca2+-gated K+ channel KCNN4 (KCa3.1). Electrophysiological and pharmacological experiments in acute brain slices showed that KCNN4-transduced cells exhibited a Ca2+-dependent slow afterhyperpolarization that significantly decreased the ability of KCNN4-positive neurons to generate high-frequency spike trains without affecting their lower-frequency coding ability and action potential shapes. Antiepileptic activity tests showed potent suppression of pharmacologically induced seizures in vitro at both single cell and local field potential levels with decreased spiking during ictal discharges. Taken together, our findings strongly suggest that the AAV-based expression of the KCNN4 channel in excitatory neurons is a promising therapeutic intervention as gene therapy for epilepsy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data supporting the key findings of this study are available within the article or from corresponding authors on request.
References
Drew L. Gene therapy targets epilepsy. Nature. 2018;564:S10–s11.
Bernard C. Treating Epilepsy with a Light Potassium Diet. Sci Transl Med. 2012;4:161fs40–161fs40.
Nikitin ES, Vinogradova LV. Potassium channels as prominent targets and tools for the treatment of epilepsy. Expert Opin Ther Targets. 2021;25:223–35.
Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4:161ra152.
Snowball A, Chabrol E, Wykes RC, Shekh-Ahmad T, Cornford JH, Lieb A, et al. Epilepsy Gene Therapy Using an Engineered Potassium Channel. J Neurosci. 2019;39:3159–69.
Magloire V, Cornford J, Lieb A, Kullmann DM, Pavlov I. KCC2 overexpression prevents the paradoxical seizure-promoting action of somatic inhibition. Nat Commun. 2019;10:1225.
Agostinho AS, Mietzsch M, Zangrandi L, Kmiec I, Mutti A, Kraus L, et al. Dynorphin-based “release on demand” gene therapy for drug-resistant temporal lobe epilepsy. EMBO Mol Med. 2019;11:e9963.
Qiu Y, O’Neill N, Maffei B, Zourray C, Almacellas-Barbanoj A, Carpenter JC, et al. On-demand cell-autonomous gene therapy for brain circuit disorders. Science (New York, N.Y.). 2022;378:523–32.
Kohling R, Wolfart J. Potassium Channels in Epilepsy. Cold Spring Harb Perspect Med. 2016;6:a022871.
Masnada S, Hedrich UBS, Gardella E, Schubert J, Kaiwar C, Klee EW, et al. Clinical spectrum and genotype-phenotype associations of KCNA2-related encephalopathies. Brain. 2017;140:2337–54.
Muona M, Berkovic SF, Dibbens LM, Oliver KL, Maljevic S, Bayly MA, et al. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat Genet. 2015;47:39–46.
Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, et al. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–53.
Corbett MA, Bellows ST, Li M, Carroll R, Micallef S, Carvill GL, et al. Dominant KCNA2 mutation causes episodic ataxia and pharmacoresponsive epilepsy. Neurology. 2016;87:1975–84.
Simons C, Rash LD, Crawford J, Ma L, Cristofori-Armstrong B, Miller D, et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat Genet. 2015;47:73–77.
Lee H, Lin MC, Kornblum HI, Papazian DM, Nelson SF. Exome sequencing identifies de novo gain of function missense mutation in KCND2 in identical twins with autism and seizures that slows potassium channel inactivation. Hum Mol Genet. 2014;23:3481–9.
Leo A, Citraro R, Constanti A, De Sarro G, Russo E. Are big potassium-type Ca(2+)-activated potassium channels a viable target for the treatment of epilepsy? Expert Opin Ther Targets. 2015;19:911–26.
Schorge S, Walker MC, Kullmann DM, Snowball A, Chabrol E. Expression vectors comprising engineered genes. WIPO (PCT). UK: Ucl Business Plc, 2018. (PCT) W, (ed).
Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–47.
Foust AJ, Yu Y, Popovic M, Zecevic D, McCormick DA. Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons. J Neurosci. 2011;31:15490–8.
Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–5.
Roshchin MV, Matlashov ME, Ierusalimsky VN, Balaban PM, Belousov VV, Kemenes G, et al. A BK channel-mediated feedback pathway links single-synapse activity with action potential sharpening in repetitive firing. Sci Adv. 2018;4:eaat1357.
King B, Rizwan AP, Asmara H, Heath NC, Engbers JD, Dykstra S, et al. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep. 2015;11:175–82.
Roshchin MV, Ierusalimsky VN, Balaban PM, Nikitin ES. Ca(2+)-activated KCa3.1 potassium channels contribute to the slow afterhyperpolarization in L5 neocortical pyramidal neurons. Sci Rep. 2020;10:14484.
Tiwari MN, Mohan S, Biala Y, Yaari Y. Differential contributions of Ca(2+) -activated K(+) channels and Na(+) /K(+) -ATPases to the generation of the slow afterhyperpolarization in CA1 pyramidal cells. Hippocampus. 2018;28:338–57.
Guan D, Armstrong WE, Foehring RC. Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca(2)(+) dependence and differential modulation by norepinephrine. J Neurophysiol. 2015;113:2014–32.
Vigneault P, Parent S, Kanda P, Michie C, Davis DR, Nattel S. Electrophysiological engineering of heart-derived cells with calcium-dependent potassium channels improves cell therapy efficacy for cardioprotection. Nat Commun. 2021;12:4963.
Bulk E, Ay AS, Hammadi M, Ouadid-Ahidouch H, Schelhaas S, Hascher A, et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int J Cancer. 2015;137:1306–17.
Turner KL, Honasoge A, Robert SM, McFerrin MM, Sontheimer H. A proinvasive role for the Ca(2+) -activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62:971–81.
Du Y, Song W, Chen J, Chen H, Xuan Z, Zhao L, et al. The potassium channel KCa3.1 promotes cell proliferation by activating SKP2 and metastasis through the EMT pathway in hepatocellular carcinoma. Int J Cancer. 2019;145:503–16.
Wang ZH, Shen B, Yao HL, Jia YC, Ren J, Feng YJ, et al. Blockage of intermediate-conductance-Ca(2+) -activated K(+) channels inhibits progression of human endometrial cancer. Oncogene. 2007;26:5107–14.
Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161:441–50.
Franklin KBJ. The mouse brain in stereotaxic coordinates / Keith B.J. Franklin, George Paxinos. Amsterdam: Elsevier; 2008.
Aseyev N, Roshchin M, Ierusalimsky VN, Balaban PM, Nikitin ES. Biolistic delivery of voltage-sensitive dyes for fast recording of membrane potential changes in individual neurons in rat brain slices. J Neurosci Methods. 2013;212:17–27.
Ilin V, Malyshev A, Wolf F, Volgushev M. Fast computations in cortical ensembles require rapid initiation of action potentials. J Neurosci. 2013;33:2281–92.
Nikitin ES, Bal NV, Malyshev A, Ierusalimsky VN, Spivak Y, Balaban PM, et al. Encoding of High Frequencies Improves with Maturation of Action Potential Generation in Cultured Neocortical Neurons. Front Cell Neurosci. 2017;11:28.
Ierusalimsky VN, Balaban PM, Nikitin ES. Nav1.6 but not KCa3.1 channels contribute to heterogeneity in coding abilities and dynamics of action potentials in the L5 neocortical pyramidal neurons. Biochem Biophys Res Commun. 2022;615:102–8.
Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–51.
Ilin V, Stevenson IH, Volgushev M. Injection of Fully-Defined Signal Mixtures: A Novel High-Throughput Tool to Study Neuronal Encoding and Computations. PLOS ONE. 2014;9:e109928.
Guo C, Peng J, Zhang Y, Li A, Li Y, Yuan J, et al. Single-axon level morphological analysis of corticofugal projection neurons in mouse barrel field. Scientific Rep. 2017;7:2846.
Baker A, Kalmbach B, Morishima M, Kim J, Juavinett A, Li N, et al. Specialized Subpopulations of Deep-Layer Pyramidal Neurons in the Neocortex: Bridging Cellular Properties to Functional Consequences. J Neurosci. 2018;38:5441–55.
Sahu G, Turner RW. The Molecular Basis for the Calcium-Dependent Slow Afterhyperpolarization in CA1 Hippocampal Pyramidal Neurons. Front Physiol. 2021;12:759707.
Jenkins DP, Yu W, Brown BM, Lojkner LD, Wulff H. Development of a QPatch automated electrophysiology assay for identifying KCa3.1 inhibitors and activators. Assay Drug Dev Technol. 2013;11:551–60.
Kondgen H, Geisler C, Fusi S, Wang XJ, Luscher HR, Giugliano M. The dynamical response properties of neocortical neurons to temporally modulated noisy inputs in vitro. Cereb Cortex. 2008;18:2086–97.
Higgs MH, Spain WJ. Conditional bursting enhances resonant firing in neocortical layer 2-3 pyramidal neurons. J Neurosci. 2009;29:1285–99.
Proskurina EY, Chizhov AV, Zaitsev AV. Optogenetic Low-Frequency Stimulation of Principal Neurons, but Not Parvalbumin-Positive Interneurons, Prevents Generation of Ictal Discharges in Rodent Entorhinal Cortex in an In Vitro 4-Aminopyridine Model. Int J Mol Sci. 2022;24:195.
Soboleva EB, Amakhin DV, Sinyak DS, Zaitsev AV. Modulation of seizure-like events by the small conductance and ATP-sensitive potassium ion channels. Biochem Biophys Res Commun. 2022;623:74–80.
Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy. A quantitative MRI study. 1999;52:1870.
Kuhn T, Gullett JM, Boutzoukas AE, Bohsali A, Mareci TH, FitzGerald DB, et al. Temporal lobe epilepsy affects spatial organization of entorhinal cortex connectivity. Epilepsy Behav. 2018;88:87–95.
Proskurina EY, Zaitsev AV. Regulation of Potassium and Chloride Concentrations in Nervous Tissue as a Method of Anticonvulsant Therapy. J Evol Biochem Physiol. 2022;58:1275–92.
Nikitin ES, Balaban PM, Zaitsev AV. Prospects for Gene Therapy of Epilepsy Using Calcium-Acivated Potassium Channel Vectors. J Evol Biochem Physiol. 2022;58:1065–74.
Walker MC, Kullmann DM. Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology 2019;168:107751.
Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. Silencing Neurons: Tools, Applications, and Experimental Constraints. Neuron. 2017;95:504–29.
Schiller Y. Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J Neurophysiol. 2004;92:862–72.
Somarowthu A, Goff KM, Goldberg EM. Two-photon calcium imaging of seizures in awake, head-fixed mice. Cell Calcium. 2021;96:102380.
Nagarkatti N, Deshpande LS, DeLorenzo RJ. Development of the calcium plateau following status epilepticus: role of calcium in epileptogenesis. Expert Rev Neurother. 2009;9:813–24.
Powell KL, Cain SM, Snutch TP, O’Brien TJ. Low threshold T-type calcium channels as targets for novel epilepsy treatments. Br J Clin Pharmacol. 2014;77:729–39.
Rajakulendran S, Hanna MG. The Role of Calcium Channels in Epilepsy. Cold Spring Harb Persp Med. 2016;6:a022723.
Higham J, Sahu G, Wazen RM, Colarusso P, Gregorie A, Harvey BSJ, et al. Preferred Formation of Heteromeric Channels between Coexpressed SK1 and IKCa Channel Subunits Provides a Unique Pharmacological Profile of Ca(2+)-Activated Potassium Channels. Mol Pharmacol. 2019;96:115–26.
Chen L, Cummings KA, Mau W, Zaki Y, Dong Z, Rabinowitz S, et al. The role of intrinsic excitability in the evolution of memory: Significance in memory allocation, consolidation, and updating. Neurobiol Learning Memory. 2020;173:107266.
Ohno M, Sametsky EA, Silva AJ, Disterhoft JF. Differential effects of alphaCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur J Neurosci. 2006;23:2235–40.
Oh MM, Simkin D, Disterhoft JF. Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits. Front Syst Neurosci. 2016;10:52.
Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging cell. 2007;6:327–36.
Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 2006;29:587–99.
Tiwari MN, Mohan S, Biala Y, Yaari Y. Protein Kinase A-Mediated Suppression of the Slow Afterhyperpolarizing KCa3.1 Current in Temporal Lobe Epilepsy. J Neurosci. 2019;39:9914–26.
Acknowledgements
This research was funded by Russian Science Foundation; grant: RSF 20-15-00408. We would like to thank Dr. Alexander S. Chernyshev for his help in analyzing the field recordings.
Author information
Authors and Affiliations
Contributions
Conceptualization, ESN, VVB, PMB, and AVZ; Funding acquisition, PMB; ESN, MVR, TYP, VI, IK, AAB, EYP, MPS, and AVZ performed the experiments; Methodology, AAB, ESN, TYP, and AVZ; Project administration, ESN, PMB, and AVZ; Writing—original draft, ESN, TYP, EYP, and AVZ; Reviewing & editing, ESN, PMB, VVB, and AVZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikitin, E.S., Postnikova, T.Y., Proskurina, E.Y. et al. Overexpression of KCNN4 channels in principal neurons produces an anti-seizure effect without reducing their coding ability. Gene Ther 31, 144–153 (2024). https://doi.org/10.1038/s41434-023-00427-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-023-00427-9