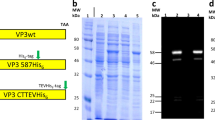
Abstract
Naturally occurring adeno-associated virus (AAV) serotypes that bind to ligands such as AVB sepharose or heparin can be purified by affinity chromatography, which is a more efficient and scalable method than gradient ultracentrifugation. Wild-type AAV8 does not bind effectively to either of these molecules, which constitutes a barrier to using this vector when a high throughput design is required. Previously, AAV8 was engineered to contain a SPAKFA amino acid sequence to facilitate purification using AVB sepharose resin; however, in vivo studies were not conducted to examine whether these capsid mutations altered the transduction profile. To address this gap in knowledge, a mutant AAV8 capsid was engineered to bind to AVB sepharose and heparan sulfate (AAV8-AVB-HS), which efficiently bound to both affinity columns, resulting in elution yields of >80% of the total vector loaded compared to <5% for wild-type AAV8. However, in vivo comparison by intramuscular, intravenous, and intraperitoneal vector administration demonstrated a significant decrease in AAV8-AVB-HS transduction efficiency without alteration of the transduction profile. Therefore, although it is possible to engineer AAV capsids to bind various affinity ligands, the consequences associated with mutating surface exposed residues have the potential to negatively impact other vector characteristics including in vivo potency and production yield. This study demonstrates the importance of evaluating all aspects of vector performance when engineering AAV capsids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–105.
Kay MA, Nakai H. Looking into the safety of AAV vectors. Nature. 2003;424:251.
Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J, et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25:180–8.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65.
Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–24.
Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20:1–16.
Smalley E. First AAV gene therapy poised for landmark approval. Nat Biotechnol. 2017;35:998–9.
Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs. 2019;79:1255–62.
Morrison C. $1-million price tag set for Glybera gene therapy. Nat Biotechnol. 2015;33:217–8.
Auricchio A, O’Connor E, Hildinger M, Wilson JM. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther. 2001;4:372–4.
Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, et al. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77:11072–81.
Xie Q, Lerch TF, Meyer NL, Chapman MS. Structure-function analysis of receptor-binding in adeno-associated virus serotype 6 (AAV-6). Virology. 2011;420:10–9.
Lerch TF, O’Donnell JK, Meyer NL, Xie Q, Taylor KA, Stagg SM, et al. Structure of AAV-DJ, a retargeted gene therapy vector: cryo-electron microscopy at 4.5 A resolution. Structure. 2012;20:1310–20.
Wang Q, Lock M, Prongay AJ, Alvira MR, Petkov B, Wilson JM. Identification of an adeno-associated virus binding epitope for AVB sepharose affinity resin. Mol Ther Methods Clin Dev. 2015;2:15040.
Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol. 2006;80:11393–7.
Srivastava A, Zhong L, Zolotukhin S, Aslanidi GV, Agbandje-McKenna M, Van Vliet KM, et al. Capsid-modified, raav3 vector compositions and uses in gene therapy of human liver cancer. US Patent. 2018:0223312 A1.
Woodard K, Samulski RJ. Methods and composition for targeted gene transfer. Patent. 2018:WO2018/035213Al.
Woodard KT, Liang KJ, Bennett WC, Samulski RJ. Heparan sulfate binding promotes accumulation of intravitreally delivered adeno-associated viral vectors at the retina for enhanced transduction but weakly influences tropism. J Virol. 2016;90:9878–88.
Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol. 2013;4:341.
Xing M, Wang X, Chi Y, Zhou D. Gene therapy for colorectal cancer using adenovirus-mediated full-length antibody, cetuximab. Oncotarget. 2016;7:28262–72.
Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–4.
Lock M, Alvira M, Vandenburghe LH, Samanta A, Toelen J, Debyser Z, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010;21:1259–71.
Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Met. 2011;23:18–28.
van Lieshout LP, Domm JM, Wootton SK. AAV-Mediated Gene Delivery to the Lung. Methods Mol Biol. 2019;1950:361–72.
van Lieshout LP, Domm JM, Rindler TN, Frost KL, Sorensen DL, Medina SJ, et al. A novel triple-mutant AAV6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol Ther Methods Clin Dev. 2018;9:323–9.
Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–24.
van Lieshout LP, Soule G, Sorensen D, Frost KL, He S, Tierney K, et al. Intramuscular adeno-associated virus-mediated expression of monoclonal antibodies provides 100% protection against ebola virus infection in mice. J Infect Dis. 2018;217:916–25.
Greig JA, Peng H, Ohlstein J, Medina-Jaszek CA, Ahonkhai O, Mentzinger A, et al. Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS ONE. 2014;9:e112268.
Ruzo A, Garcia M, Ribera A, Villacampa P, Haurigot V, Marco S, et al. Liver production of sulfamidase reverses peripheral and ameliorates CNS pathology in mucopolysaccharidosis IIIA mice. Mol Ther. 2012;20:254–66.
Wolf DA, Hanson LR, Aronovich EL, Nan Z, Low WC, Frey WH 2nd, et al. Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration. Mol Genet Metab. 2012;106:131–4.
Murillo O, Luqui DM, Gazquez C, Martinez-Espartosa D, Navarro-Blasco I, Monreal JI, et al. Long-term metabolic correction of Wilson’s disease in a murine model by gene therapy. J Hepatol. 2016;64:419–26.
Gigout L, Rebollo P, Clement N, Warrington KH Jr., Muzyczka N, Linden RM, et al. Altering AAV tropism with mosaic viral capsids. Mol Ther. 2005;11:856–65.
Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74:8635–47.
Tseng YS, Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front Immunol. 2014;5:9.
Nass SA, Mattingly MA, Woodcock DA, Burnham BL, Ardinger JA, Osmond SE, et al. Universal method for the purification of recombinant AAV vectors of differing serotypes. Mol Ther Methods Clin Dev. 2018;9:33–46.
Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–10.
Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, et al. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS ONE. 2013;8:e67680.
Foust KD, Poirier A, Pacak CA, Mandel RJ, Flotte TR. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Gene Ther. 2008;19:61–70.
Acknowledgements
We thank all those involved in the care of the animals for these studies at all institutions.
Funding
Operating funds were from the Canadian Institute of Health Research (SKW—grant # 352532), the Canadian Lung Association (SKW—grant # 052919), and the National Institute of Health (JPB—HL131634). LPvL was funded by an Ontario Graduate Scholarship. AAS was funded by a Vanier Canada Graduate Scholarship, Brock Doctoral Scholarship, and the Ethel Rose Charney Scholarship in the Human/Animal Bond.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were approved by the institutional Animal Care Committees at the University of Guelph, the Cincinnati Children’s Hospital Medical Center, and the National Microbiology Laboratory, in accordance with the Canadian Council on Animal Care guidelines (AUPP 3827). Six to eight-week-old female BALB/c mice were purchased from Charles River Laboratories (St. Constant, Quebec, Canada) and allowed to acclimatize 1 week prior to experimentation. All mice were healthy and criteria was established prior to ordering mice. Mice were of the same age, sex, and shipment. Thus, no further randomization was conducted aside from randomly selecting one cage per experimental group. The investigator was not blinded during the experiment as this study was analyzing expression as quantified objectively by a machine, and not subjective parameters such as survival endpoint in a disease model.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Lieshout, L.P., Stegelmeier, A.A., Rindler, T.N. et al. Engineered AAV8 capsid acquires heparin and AVB sepharose binding capacity but has altered in vivo transduction efficiency. Gene Ther 30, 236–244 (2023). https://doi.org/10.1038/s41434-020-00198-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00198-7