Abstract
Background/objectives
We aimed to measure cerebrospinal fluid (CSF) flow rates in the subarachnoid space (SAS) of the optic nerve (ON) by applying non-invasive diffusion-weighted MRI in patients with normal tension glaucoma (NTG) compared to age-matched controls.
Subjects/methods
In this prospective study, an analysis of diffusion-weighted images of 26 patients with NTG (49ONs) and age-matched volunteers (52ONs) was conducted. Subjects were classified into 4 groups: group I (50–59 y., n = 12 eyes), group II (60–69 y., n = 16 eyes), group III (70–79 y., n = 18 eyes) and group IV ( > 80 y., n = 6 eyes) for NTGs and healthy volunteers, respectively. The flow-range ratio (FRR) between the frontal lobe SAS and the SAS of the ON was calculated for each age category group and then compared between age-categories as well as between NTGs and controls.
Results
The mean FRR for age groups were (I) 0.54 ± 0.06 and 0.62 ± 0.03 (p < 0.05), (II) 0.56 ± 0.08 and 0.63 ± 0.03 (p < 0.05), (III) 0.54 ± 0.06 and 0.62 ± 0.02 (p < 0.001) as well as (IV) 0.61 ± 0.03 and 0.61 ± 0.04, for NTGs and controls, respectively. Using pooled data, the difference between the FRR in NTGs and controls was statistically significant (p < 0.0001). There were no statistically significant differences within the age categories of the control group. When comparing the FRR of NTGs by age categories, no statistically significant difference was found between the subgroups.
Conclusions
FRR was significantly reduced in NTGs compared to age-matched controls without any significant differences within the age groups themselves. Given the physiological importance of CSF for the integrity of neurons, axons and glial cells, reduced CSF flow dynamics might be part of the underlying neurodegenerative process of NTG.
Similar content being viewed by others
Introduction
Normal tension glaucoma (NTG) is thought to be a variant of primary open angle glaucoma (POAG). It is estimated to make up to 40% of POAG in the western hemisphere [1] and up to 90% in Asian countries [2]. NTG, like open angle glaucoma is more common in the elderly and rather unusual in patients younger than 50 years of age [3]. The average reported age in clinical studies is generally in the 60 s. Epidemiologically, age is therefore one of the main risk factors for NTG [4].
The role of intraocular pressure (IOP) in NTG is not well understood but lowering IOP has been shown to somehow slow the progression of visual field loss [5]. However, even after successful IOP lowering, retinal ganglion cell loss with consecutive visual field defects is progressive in many patients. To explain this phenomenon other pathophysiological mechanisms were introduced, among them vascular dysregulation, a higher than normal pressure gradient across the lamina cribrosa, glymphatic stasis within the optic nerve and compartmentation of cerebrospinal fluid (CSF) within the subarachnoid space (SAS) of the optic nerve [6, 7].
CSF functions as a transport system that delivers vital substances such as neurotransmitters, hormones, minerals and nutrients to the neuronal tissue such as neurons, axons and glial cells. On the other hand, it removes toxic metabolites from the parenchyma, among them abetalipoprotein and alfasynuclein from the parenchyma. Disturbed CSF dynamics lead to an undersupply of neurotrophic factors and to an accumulation of toxic biological substances [8, 9].
Recent studies [10] using cisternography and diffusion-weighted magnet resonance imaging (DWI MRI) [11] demonstrated decreased CSF flow along the ON in patients with NTG. This finding indicates that the CSF flow and thus CSF turnover along the optic nerve (ON) can become reduced in patients with NTG. Several pathophysiological processes, such as inflammation, elevated pressure and other age-related degenerative processes can cause a remodelling of the subarachnoid space (like arteriosclerotic changes in blood vessels) resulting in compartmentalisation of the SAS of the ON [12].
DWI provides a non-invasive measurement of the movement of molecules, mainly water in biological tissues. Using phase contrast images, the phase shift can be used to determine the flow velocities of coherently using particles [13, 14].
The aim of this study was to measure CSF flow rates in the SAS of the ON by applying non-invasive diffusion weighted MRI in patients with NTG compared to age-matched non glaucoma controls. Patients and controls were divided in age groups in order to investigate a possible influence of age on CSF flow rates.
Materials and methods
This monocentric, prospective observational study was approved by the local ethical commission and followed the tenets of the Declaration of Helsinki. All studied individuals, age matched healthy controls, (n = 52 eyes, age range from 50 to 87 years) and NTG patients (n = 49 eyes, age range from 51 to 84 years), gave their written informed consent.
Subjects
NTG was diagnosed on the basis of glaucomatous optic disc morphology and concomitant visual field defects with IOP always (without treatment) < 21 mmHg. Glaucomatous changes included optic disc cupping, with or without notches in the neuroretinal rim and localised or segmental loss of retinal nerve fibre layer. Visual field defects were shown by standard automated perimetry (SAP) (Programme G2, Octopus Haag-Streit, Switzerland).
Each patient underwent full ophthalmologic examination including slit lamp assisted biomicroscopy, applanation tonometry, gonioscopy, measurement of central corneal thickness and neuroretinal rim assessment with Heidelberg OCT (HEYEX, red sector on colour code compared to normative database, Heidelberg engineering, CA, USA). None of the patients had a refractive error > +3 or < −3 dioptres (D). 8/26 patients (6 bilateral, 2 unilateral) had IOP lowering drops to keep the IOP as low as possible. 12/26 patients underwent cataract surgery (10 bilateral, 2 unilateral) and none of them had glaucoma surgery. The mean glaucomatous visual field defect (MD) at time of MRI was 12 ± 6 dB and the mean IOP 12 ± 3 mmHg. None of the included NTG patients suffered from any other eye disease or underwent previous posterior segment surgery that may affect the visual field.
Controls
All control subjects were volunteers and none of the controls had glaucomatous optic nerve morphology, nor were they on anti-glaucomatous treatment. For each healthy control, the anatomical MRI scan of the ON was normal.
All subjects (NTG and controls) were classified into 4 groups: group I (50–59 years old; nNTG = 10 ONs, mean age: 53 ± 1 years, 6 males; ncontrols = 12 ONs, 54 ± 3 years, 3 females), group II (60–69 years old; nNTG = 15 ONs, mean age: 65 ± 3 years, 6 females; ncontrols = 16 ONs, 64 ± 3 years, 5 females), group III (70–79 years old; nNTG = 18 ONs, mean age: 74 ± 3 years, 7 females; ncontrols = 18 ONs, 75 ± 3 years, 4 females) and group IV ( > 80 years old; nNTG = 6 ONs, mean age: 83 ± 1 years, 3 males; ncontrols = 6 ONs, 86 ± 2 years, 1 female) (Table 1).
The flow velocity between the frontal lobe SAS and the SAS of the ON was calculated and presented in ratios. This was then compared between NTG’s and control’s age-categories.
MRI
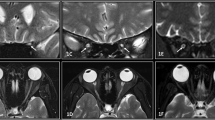
Images were acquired with a 3 T whole body magnet (Skyra; Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil using Stejskal-Tanner diffusion sequence using following parameters: b = 50 s/mm2, TE/TR = 65/2000 ms, 6 slices, 1 mm slice thickness with acquisition time of 4.13 min, each slice acquired 120 times. Estimating the flow velocities of coherent moving particles through phase contrast images is described in detail in Boye et al. [11]. In-house code programmed in Matlab (MathWorks) was used for image analysis [11]. Shortly, the monopolar diffusion gradients of the diffusion sequence led to a constant phase shift for coherently moving particles. By using the b-values, the maximum encoded velocity (venc) before a phase wrap occurs can be solved and the phase shift can then be used to determine the flow velocity range. Since the phase shift of the diffusion sequence is highly irregular, results are presented as Flow Range Ratio (FRR), which allows the easy comparison between different groups.
Statistics
Statistical analysis was performed with unpaired t-test performed by SPSS Statistics Software version 21 (IBM Corporation, Armonk, NY, USA) to compare the FRR results between the (i) NTG patients and healthy controls within the age groups, (ii) NTG patients within the age groups as well as (iii) healthy controls within the age groups. For both groups, every second OD and every second OS ON was chosen for statistics.
Results
The mean FRR for age groups, when considering 1 eye per person, were (I) 0.54 ± 0.06 (95% CI [0.42, 0.66]) and 0.62 ± 0.03 (95% CI [0.56, 0.68]), n = 6, p < 0.05, (II) 0.56 ± 0.08 (95% CI [0.40, 0.72]) and 0.63 ± 0.03 (95% CI [0.57, 0.69], n = 8, p < 0.05), (III) 0.54 ± 0.06 (95% CI [0.42, 0.66]) and 0.62 ± 0.02 (95% CI [0.58, 0.66], n = 9, p < 0.001) and (IV) 0.61 ± 0.03 (95% CI [0.55, 0.68]) and 0.61 ± 0.04 (95% CI [0.53, 0.69]), for patients with NTG and healthy controls, respectively (statistically significant difference given in brackets: Fig. 1, Tables 1 and 2).
Subgroup comparison between patients with NTG (n = 26) and age-matched healthy volunteers (n = 26) based on their age: group I (50–59 years old, n = 6 eyes), group II (60–69 years old, n = 8 eyes), group III (70–79 years old, n = 9 eyes) and group IV ( > 80 years old, n = 3 eyes). Statistically significant difference marked with *(p < 0.05)/**(p < 0.001) between the FRR of NTG patients when compared to the healthy volunteers. Group IV is left out of the statistical comparisons due to a small subject size.
For pooled data (including all the age groups), the difference between the FRR in NTG patients (0.55 ± 0.06, 95% CI [0.43, 0.67]) and healthy controls (0.62 ± 0.03, 95% CI [0.56, 0.68]) was statistically significant (n = 26, p < 0.0001).
When comparing the FRR by age categories no statistically significant difference was found between the age categories neither for NTG patients nor for the healthy controls. Groups IV were left out of the statistical comparisons due to a small subject size.
Discussion
This study performed with diffusion weighted MRI demonstrates a significantly reduced CSF flow range ratio (FRR) in patients with NTG compared to age-matched controls without a history of glaucoma. While the CSF flow range in NTGs was the same in the age groups I, II and III and greater in group IV, in age-matched healthy controls a slight decrease from group I to IV was observed.
Calculation of the flow range ratio (FRR) on diffusion-weighted MRI scans between the SAS of the frontal lobe and the SAS of the SAS of the ON reflects the flow velocity of the CSF. These measurements therefore allow the assessment of CSF flow dynamics in the SAS of the ON in a non-invasive way.
The pathophysiology of the optic neuropathy in NTG is poorly understood. Although reduction of intraocular pressure can slow the progression of the disease, a substantial number of patients progress despite successfully lowered IOP [5]. Alternative pathophysiological explanations are therefore under discussion [4, 7]. Several recent studies indicated disturbed CSF dynamics in the SAS of the ON with pathological changes in pressure and CSF composition [7, 10]. In a large series of NTG patients using computer tomography assisted cisternography a gradual reduction of contrast loaded CSF from intracranially to the bulbar portion of the ON behind the lamina cribrosa was demonstrated in contrast to the control group [10]. In accordance with these findings the current study demonstrates a reduced CSF flow velocity in the SAS of the ON in the NTG group compared to a non NTG cohort.
At least two possible mechanisms can explain reduced CSF flow within the SAS along the ON in NTG patients: (i) compartmentalisation of the optic nerve sheath and (ii) low CSF pressure. The ON SAS is bridged on its whole length with trabeculae and septae [15]. These anatomical structures as well as the pia and the arachnoid layer are covered with meningothelial cells which display mechanosensitive characteristics. They proliferate and growth if the external pressure is being elevated or if oxidative stress is applied [16]. Such growth and proliferation lead to a remodelling of the SAS and a reduction of the free space that allows CSF to flow. The CSF pressure within an optic nerve sheath compartment is unknown but, due to its compartmentalisation, likely independent from that intracranially. Low intracranial pressure, as suggested by some groups who have measured lower lumbar CSF pressure in patients with NTG, may also play a role [17]. Decreased CSF pressure directed from the basal cistern to the ON SAS, particularly in combination with a narrower OC, could therefore be another explanation for lower CSF flow velocity within the ON SAS in NTG patients. However, whether the intracranial CSF pressure is reduced in patients with NTG remains controversial [18].
In the current study, the CSF flow range in NTG patients was the same in the age groups from 50 to 79 years (groups I, II and III) while it was larger in the age group over 80 years (group IV). If optic nerve sheath compartmentalisation with impaired CSF turnover plays a role in the pathophysiology of NTG, the severity of compartmentalisation may be the most important factor. The exact mechanism leading to ON sheath compartment is however not yet revealed. As it is increasingly found in patients with elevated CSF pressure and in patients with a history of meningitis, age-independent pressure gradients and inflammatory processes seem to play a role [19]. The larger CSF flow range in NTG patients over 80 may be explained by the small number (n = 3) of patients in this group.
There is a growing body of evidence that CSF dynamics seems to become impaired with age which plays a crucial role in brain health in older people [20]. However, in the current study we did not find a clear decrease of the CSF flow range with age, which may be due to the relatively small numbers in each age group. There are only few studies that have systematically examined CSF pressure and age. While some studies [21] did not find a relationship between age and CSF pressure, a retrospective study demonstrated in 12,118 patients a sustained and significant reduction of CSF pressure beginning in the 6th decade [22]. A lower CSF pressure will consequently lead to a lower CSF flow velocity. CSF pressure is usually measured by lumbar puncture. As lumbar puncture is performed quite remote from the ON SAS it is not clear whether the lumbar CSF pressure equals the CSF pressure in the ON SAS and whether the CSF pressure is lower at all in the older controls.
Adequate CSF flow is essential for the delivery of neurotropic factors, electrolytes, neurotransmitters and hormones to neurons, axons, and glial cells in the central nervous system (CNS). However, its role as a clearing system of the CNS is just as important. Several neurodegenerative diseases, such as Alzheimers disease, Parkinsons disease, Lew-body -dementia and frontotemporal dementia are linked to a reduced clearage of abetalipoprotein, alfasynuclean or Tauproteins and other biomarkers [23]. Stagnant CSF causes an accumulation of such biomarkers of neurodegeneration. CSF samples from a compartmented ON SAS of NTG patients have demonstrated elevated levels of L-PGDS (a multifunctional prostaglandine synthetase), indicating CSF stagnation in the ON SAS [24]. However, future studies need to investigate how and to what extent CSF stagnation in the optic nerve SAS leads to the optic nerve damage as seen in patients with NTG.
There are some limitations of this study: First, as the CSF flow within the ON is dependent on the RR interval in ECG-curve of the heart, the RR interval was used to report our results as ‘flow range’, and not as flow, since the phase offset. Ratio is not dependent on the offset, therefore, making it possible to compare values between the measurements. Second, the number of subjects was relatively small in the age-categorised subgroups, particularly in group IV, which was therefore left out of the statistical comparisons.
In summary, CSF flow range was significantly reduced in patients with NTG compared to age-matched non glaucomatous controls. CSF flow studies applying non-invasive flow rate measuring could be used as an addition to intraocular pressure measurement in therapy refractive NTG patients.
Summary
What was known before
-
Age is one of the main risk factors for NTG.
-
CSF flow and thus CSF turnover along the optic nerve can become reduced in patients with NTG.
-
Patients and controls were divided in age groups in order to investigate a possible influence of age on CSF flow rates.
What this study adds
-
CSF Flow was significantly reduced in NTGs in different age categories compared to age-matched controls without any significant differences within the age groups themselves.
-
Reduced CSF flow dynamics might be part of the underlying neurodegenerative process of NTG.
Data availability
Data is freely available for non-commercial purposes and can be requested from the corresponding author.
References
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59:434–47.
Park HL, Shin DY, Jeon SJ, Kim YC, Jung Y, Kim EK, et al. Predicting the development of normal tension glaucoma and related risk factors in normal tension glaucoma suspects. Sci Rep. 2021;11:16697.
Mi XS, Yuan TF. The current research status of normal tension glaucoma –review. Clin Interv Aging. 2014;16:1563–71.
The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. [No authors listed]. Am J Ophthalmol (1998) 126, 498–505.
Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance – new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021;99:e283–e284.
Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye. 2018;32:924–30.
Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–5.
Chung CG, Lee H, Lee SB. Mechanisms of protein toxicity in neurodegenerative diseases. Cell Mol Life Sci. 2018;75:3159–80.
Pircher A, Montali M, Wostyn P, Pircher J, Berberat J, Remonda L, et al. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018;96:e562–e569.
Boye D, Montali M, Miller NR, Pircher A, Gruber P, Killer HE, et al. Flow dynamics of cerebrospinal fluid between the intracranial cavity and the subarachnoid space of the optic nerve measured with a diffusion magnetic resonance imaging sequence in patients with normal tension glaucoma. Clin Exp Ophthalmol. 2018;46:511–8.
Killer HE, Subramanian PS. Compartmentalized cerebrospinal fluid. Int Ophthalmol Clin. 2014;54:95–102.
Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, et al. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol. 2005;15:2172–84.
Søndergaard L, Lindvig K, Hildebrandt P, Thomsen C, Ståhlberg F, Joen T, et al. Quantification of aortic regurgitation by magnetic resonance velocity mapping. Am Heart J. 1993;125:1081–90.
Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777–81.
Xin X, Fan B, Flammer J, Miller NR, Jaggi GP, Killer HE, et al. Meningothelial cells react to elevated pressure and oxidative stress. PLoS ONE. 2011;6:e20142.
Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010;117:259–66.
Lindén C, Qvarlander S, Jóhannesson G, Johansson E, Östlund F, Malm J, et al. Normal-tension glaucoma has normal intracranial pressure: a prospective study of intracranial pressure and intraocular pressure in different body positions. Ophthalmology. 2018;125:361–8.
Hao J, Pircher A, Miller NR, Hsieh J, Remonda L, Killer HE. Cerebrospinal fluid and optic nerve sheath compartment syndrome: a common pathophysiological mechanism in five different cases? Clin Exp Ophthalmol. 2020;48:212–9.
Hidaka Y, Hashimoto M, Suehiro T, Fukuhara R, Ishikawa T, Tsunoda N, et al. Impact of age on the cerebrospinal fluid spaces: high-convexity and medial subarachnoid spaces decrease with age. Fluids Barriers CNS. 2022;19:82.
Malm J, Jacobsson J, Birgander R, Eklund A. Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology. 2011;76:903–9.
Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR. Cerebrospinal fluid pressure decreases with older age. PLoS ONE. 2012;7:e52664.
Tarasoff-Conway J, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–70.
Pircher A, Neutzner A, Montali M, Huber A, Scholl HPN, Berberat J, et al. Lipocalin-type prostaglandin d synthase concentration gradients in the cerebrospinal fluid in normal-tension glaucoma patients with optic nerve sheath compartmentation. Eye Brain. 2021;13:89–97.
Funding
Financial support from the Swiss National Science Foundation (Grant 196877) is acknowledged.
Author information
Authors and Affiliations
Contributions
JB was responsible for designing the study, writing the manuscript, screening potentially eligible studies, extracting and analysing data, doing statistics and interpreting results. AP, LR and HK contributed to writing, interpreting results and updating reference lists.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berberat, J., Pircher, A., Remonda, L. et al. Age related cerebrospinal fluid flow dynamics in the subarachnoid space of the optic nerve in patients with normal tension glaucoma, measured by diffusion weighted MRI. Eye (2024). https://doi.org/10.1038/s41433-024-03084-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-024-03084-3