Abstract
Background/Objectives
The acceptability of emerging intravitreal therapies for patients with Geographic Atrophy (GA) is currently unknown. This study therefore aimed to investigate the extent to which regular intravitreal injections may be acceptable to GA patients.
Subjects/Methods
Thirty UK-based individuals with GA secondary to age-related macular degeneration (AMD), recruited from two London-based hospitals, were interviewed in April-October 2021 regarding acceptability of new GA treatments. Participants responded to a structured questionnaire, as well as open-ended questions in a semi-structured interview. The Theoretical Framework of Acceptability (TFA) informed framework analysis of the qualitative data.
Results
Twenty participants (67%) were female, and median (interquartile range (IQR)) age was 83 (78, 87) years. 37% of participants had foveal centre-involving GA, and better eye median (IQR) logMAR visual acuity was 0.30 (0.17, 0.58). Data suggested that 18 participants (60% (95% CI: 41–79%)) would accept the treatment, despite awareness of potential drawbacks. Eight participants (27% (95% CI: 10–43%) were ambivalent or undecided about treatment, and four (13%) (95% CI: 0–26%) would be unlikely to accept treatment. Reducing the frequency of injections from monthly to every other month increased the proportion of participants who considered the treatments acceptable. Conversely, factors limiting acceptability clustered around: the limited magnitude of treatment efficacy; concerns about side effects or the increased risk of neovascular AMD; and the logistical burden of regular clinic visits for intravitreal injections. Misunderstandings of potential benefits indicate the need for appropriately-designed patient education tools to support decision-making.
Conclusions
Our study suggests a majority of participants would be positive about intravitreal treatment for GA, in spite of potential burdens.
Similar content being viewed by others
Introduction
Geographic Atrophy (GA) is the advanced form of the non-neovascular (‘dry’) type of age-related macular degeneration (AMD), affecting 276,000 people in the UK [1]. While there are now approved treatments for wet AMD, until recently there has been no therapy for GA, a significant unmet need [2]. Even before the foveal centre is involved, GA can have significant impact on functional activities and vision-related quality-of-life [3, 4].
Dysregulation of the complement cascade has been implicated in the pathogenesis of GA, and there are now two intravitreal complement inhibitors in late-stage development for the treatment of GA [2]. Regular intravitreal injections are the standard of care for wet AMD, and a common mode of delivery in the current pipeline of treatments for GA in clinical trials. Recent positive results from phase 3 clinical trials of two intravitreal complement inhibitors provide hope for a treatment for GA [5,6,7]. Indeed, in February 2023, the first-ever treatment for GA, pegcetacoplan, was approved for use by the Food and Drug Administration (FDA) in the US under the brand name Syfovre, based on reduced rates of lesion growth in the DERBY and OAKS trials [8]. However, it is not yet known whether such treatments will be acceptable to patients outside clinical trial settings.
Current evidence from wet AMD suggests people will persevere with regular intravitreal treatment, even when associated with a high burden, when motivated by outcome expectations [9, 10]. Despite efficacious outcomes of anti-VEGF therapy [11], some wet AMD patients report significant treatment burden associated with regular intravitreal injections, not only in terms of anxiety, discomfort, pain and/or side effects associated with these injections, but also the logistics of regularly travelling to the eye clinic, waiting times, and impacts on accompanying relatives or caregivers [12,13,14].
However, GA is different to wet AMD, being slower to progress, with well-documented variation in rates of progression across individuals, and asymptomatic in some patients until involving the fovea [15, 16]. Therefore, it is vital to understand whether patients with GA would find it acceptable to commence and adhere to frequent intravitreal treatments, in order to slow GA progression.
Acceptability, as defined by Sekhon and colleagues in their Theoretical Framework of Acceptability (TFA), is a “multi-faceted construct that reflects the extent to which people delivering or receiving a healthcare intervention consider it to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention” [17]. Acceptability is a crucial yet complex factor which can have implications for patients deciding to undergo a treatment, as well as adhering and persisting with it. As such, assessment of acceptability to patients should be a critical first step in the design, evaluation and delivery of healthcare interventions [18].
Our study’s central objective was to explore the overall acceptability of current intravitreal treatments in late-stage development for a sample of GA patients. We aimed to identify which aspects of the treatment are considered less acceptable; and to understand whether specific patient-related factors, contexts and circumstances influence GA treatment acceptability. A secondary aim was to explore what people with GA understand about their disease, its progression, current service provision, and their hopes for GA treatment and/or cure.
Methods
Study design and procedure
This study employed a cross-sectional, mixed-methods design [19], and full detail on methodological aspects is presented in the published study protocol [20]. In summary, a structured questionnaire was used to quantify participants’ attitudes to acceptability, as well as open-ended questions to explore participants’ beliefs, hopes and concerns regarding GA treatment within their unique contexts and circumstances. Information communicated to participants about the treatments’ efficacy was based on Phase 2 clinical trial results [21,22,23].
The study procedure is summarised in Fig. 1. The interview schedule, including Likert-type scale questions and semi-structured open-ended questions, is shown in Appendix 1. This interview schedule was developed in consultation with a group of eight patient advisors, individuals living with GA who did not participate in this study but generously volunteered their time and insights.
The nine steps of the procedure, beginning with (1) information about GA and the new treatments, followed by (2) socio-demographic questions, (3) the EQ-5D-5L patient-reported outcome measure, (4) Likert-type scale questions about the participants' perspective on GA, (5) (re)delivery of information about the GA treatments, (6) Likert-type scale questions on acceptability of treatment, (7) open-ended, semi-structured interview questions on acceptability of treatment, (8) a discrete-choice experiment style task, to be discussed in a separate paper, and (9) semi-structured questions on the experience of living with GA.
Participant recruitment
Individuals with a diagnosis of GA were recruited from two Medical Retina clinics in London including Brent, one of the most ethnically diverse boroughs in London, UK [24]. Included participants were required to be aged ≥ 50 years, and have a diagnosis of GA (bilateral or unilateral) secondary to age-related macular degeneration. Patients with other causes of GA - such as Stargardt’s - or with concurrent retinal conditions were excluded. The aim was to recruit a cohort representative of the population in the community; therefore, some participants required an accompanying relative/caregiver to interpret parts of the interview.
In order to explore the views of participants with varied demographic and clinical characteristics, a purposive sampling strategy was employed, aiming to achieve maximum variation [25] in terms of: age; gender; ethnicity; education level; overall health status; prior experience of intravitreal injections (for wet AMD); best-corrected visual acuity (BCVA); laterality; and foveal involvement, with extrafoveal defined as greater than 0 microns from the fovea [26].
Consenting participants undertook an audio-recorded interview face-to-face or via telephone with authors AG, CD, or JE between April and October 2021. This decision to undertake certain interviews by telephone was a pragmatic response to COVID-19 restrictions in place in the UK at the time [27].
Ethical considerations
Ethics Committee approval was obtained from the NHS Health Research Authority on 23 March 2021 (IRAS Project ID: 287824), and the study adhered to the tenets of the Declaration of Helsinki.
Data analysis
Quantitative responses
Descriptive analysis of demographic information and responses to the Likert-type scale questions was undertaken. Where appropriate, Spearman’s rank (rs) correlation coefficients were calculated to explore potential associations between responses to the Likert-type scale questions on acceptability (dependent variables) and demographic and clinical characteristics (independent variables). A p-value of < 0.05 was considered statistically significant. Statistical tests were conducted using SPSS, version 27.0 (SPSS Inc., Chicago, IL, USA).
Qualitative responses
Data from the semi-structured interview were transcribed verbatim, and analysed using the Framework Method of analysis [28, 29]. This systematic qualitative data analysis method allowed for both inductive analysis (whereby open coding of the data leads to generation of themes) and deductive analysis (whereby pre-existing theory – in this case, the TFA - shapes the development of themes). Initial coding was conducted by author JE, followed by a second round of coding involving authors JE, AG, DJT and CD working collaboratively. Discrepancies regarding the best fit of text segments within the TFA matrix were resolved by author MS, an expert in acceptability who developed the TFA. This was an iterative, recursive process, and over time the team collaboratively developed a codebook (Appendix 2), establishing decision rules for coding the data into the seven TFA constructs. The software package NVIVO V.10.2 (QSR International, Cambridge, Massachusetts, USA) was used to manage the qualitative data.
In tandem, data which did not fit within a TFA construct were coded inductively by authors JE, AG, and CD, to develop a second framework matrix encapsulating important patterns in the data falling outside the TFA.
Analysis of qualitative data within the framework matrix illustrated that participants’ responses fell within three distinct and recognisable positive, ambivalent, and negative categories [30]. The categorisation was based on participants’ expressed intentions regarding the potential treatments. For example, a participant concluding that “I think I would have the treatment at almost any cost” (P26) would be placed in the positive category, while a participant concluding that the treatment “is not for me” (P24) would be placed in the negative category. Two authors (CD and JE) independently assigned the participants into the three categories, and then compared and collaboratively refined the categorisation. Certain disagreements in categorisation were discussed with reference to the individual case in the framework matrix, and all authors subsequently met to consider these disputed cases and reach consensus. After whole team discussion, the three categories were termed “Treatment at any cost” (positive), “Ambivalent”, and “Unlikely to Proceed” (negative).
Results
Participants
Thirty participants (67% female) were interviewed, and demographic and clinical characteristics for each participant are displayed in Appendix 3. Median (interquartile range (IQR)) age was 83 (78, 87) years. Nineteen (63%) of participants identified as white, eight (27%) as South Asian, one (3%) as Black, and two (7%) as another ethnicity. The range of participants’ primary languages is displayed in Appendix 4. In the case of three participants (P16, P20, and P25), interviews were interpreted by or mediated through an accompanying relative, due to English language or communication difficulties.
Better eye median (IQR) logMAR visual acuity (VA) was 0.30 (0.17, 0.58). Nineteen (63%) of the 30 participants had prior experience of intravitreal injections for neovascular (wet) AMD, while 11 (37%) were injection-naïve. Eleven (37%) of participants had centre-involving GA.
When asked to self-report their GA severity (Appendix 1, Q16), 13 participants self-rated their GA as mild, 13 as moderate, and 4 as severe. A more severe self-report was associated with worse VA in the better eye (rs (28) = 0.40, p = 0.029). This is consistent with previous reports demonstrating that vision-related quality of life is primarily dependent on the better eye [31]. However, there was no statistically significant correlation between self-reported GA severity and: worse eye VA; VA in the GA eye; VA in the fellow eye; GA laterality; or centre-involvement.
Median (IQR) time to travel to the eye clinic was 30 (15, 45) minutes. Ten (33%) participants lived alone while the other 20 (67%) lived with spouses or partners, children or carers. Fourteen (47%) participants reported attending eye clinic appointments alone, while the other 16 (53%) were accompanied by relatives, friends or caregivers. Twenty-three (77%) of participants reported living with other chronic health conditions apart from AMD/GA, with 8 (27%) living with diabetes. In the EQ-5D, the domains in which participants reported most problems were mobility (mean score = 2.3) and usual activities (mean score = 2.1).
Interview times with participants ranged from 27 min to 120 min. Twenty-four of the interviews (80%) were conducted in person, and six (20%) by telephone.
Quantitative findings on acceptability of intravitreal injections for GA
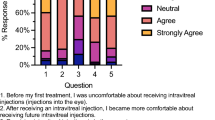
Findings from the Likert-type scale questions about acceptability of GA treatment are shown below in Table 1, while Fig. 2 displays responses to questions about participants’ willingness to undergo intravitreal injections at different intervals. Figure 2 demonstrates the increase in acceptability when injections were proposed every other month rather than monthly, with 15 of 30 (50%) participants extremely likely to accept GA injections every other month, compared with 9 of 30 (30%) extremely likely to accept monthly GA injections.
The bar chart demonstrates that as the time intervals between intravitreal injections increase, participants would be more likely to accept the injection treatments. For example, 9 participants would be extremely likely and 10 would be fairly likely to accept injections once per month. By contrast, 19 participants would be extremely likely and 4 would be fairly likely to accept injections once every six months.
Qualitative responses analysed within the TFA (see below) were additionally categorised into three groups, following analysis of the qualitative framework and reaching consensus among all authors. Eighteen (60% (95% CI: 41–79%)) participants were deemed to be positively accepting of the treatment despite their awareness of the burdens and drawbacks, and this group was termed “Treatment at any cost”. Eight (27% (95% CI: 10–43%)) participants were deemed to be “Ambivalent”, hesitant about treatment and unsure about the balance of benefits versus risks and drawbacks. Four (13% (95% CI: 0–26%)) participants were deemed “Unlikely to proceed” with treatment. These figures correlate strongly with participants’ responses on the Likert-type scale question asking whether the risks of treatment are worth the benefits (Table 1), rs (28) = 0.69, p < 0.001. Table 2 shows these acceptability levels, overall and as stratified by select ocular and demographic characteristics.
Inferential analysis demonstrated a statistically significant, moderate correlation between overall acceptability level (i.e., membership in the three groups discussed in the paragraph above) and EQ-5D score, rs (28) = 0.42, p = 0.021. Participants with worse self-reported health (higher EQ-5D score) were more likely to be in the “Treatment at any cost” group. Otherwise, there were no statistically significant associations between treatment acceptability and demographic/clinical factors, such as intravitreal injection history.
When considering correlations between other Likert-type scale question responses and demographic/clinical factors, statistically significant moderate correlations were only found for the question around concern about side effects of injections. Concern about side effects correlated positively with: increased age, rs (28) = 0.44, p = 0.014; presence of other chronic health conditions, rs (28) = 0.47, p = 0.009; and naivety to intravitreal injections, rs (28) = 0.43, p = 0.018.
Qualitative findings on acceptability of intravitreal injections for GA, based around the Theoretical framework of acceptability (TFA)
Participants’ responses to the semi-structured, open-ended interview questions were coded into the seven constructs of the TFA [17]. Table 3 displays the seven constructs as defined in the TFA, and different reflections of the construct as generated from participants’ responses, illustrated with example verbatim quotations. Appendix 5 provides an extended version of these qualitative findings, with additional participant quotations.
Qualitative findings beyond the TFA
Themes were also generated inductively from aspects of participants’ accounts that fell outside the constructs of the TFA, but were still relevant to GA treatment acceptability. These themes and associated quotations are presented in Appendix 6.
Discussion
Our study findings suggest that a majority of GA patients would be accepting of intravitreal treatment for GA, whilst recognising potential burdens and inconveniences. The key concern for people with GA, which emerged in our study as the central motivation for treatment, is the high priority placed on ability to continue with vision-specific activities, particularly for those in worse self-reported health. For 60% of the study participants, despite acknowledging potential drawbacks, the possibility of extending the time they have to engage in vision-specific activities and remain independent was deemed a worthy trade-off, and they would therefore opt for ‘treatment at any cost’. The factors limiting acceptability were largely clustered around concerns about magnitude of treatment efficacy, fear of wet AMD and side effects (and to a lesser extent, the injection procedure itself), and logistics of regular eye clinic visits for treatment. Specifically, reducing the frequency of injections from monthly to every other month increased the proportion of participants that were extremely likely to accept these treatments if offered now.
Interestingly, as explored within the TFA’s Perceived Effectiveness construct, there were a number of participants with better visual acuity than the sample average who saw no value in treatment, because they perceived their vision as currently good and thus saw no rationale for treatment. However, natural history studies demonstrate a progressive decline in vision over time, with almost two-thirds of eyes observed to have foveal involvement associated with moderate or severe sight loss within 4–5 years [16, 32]. Additionally, the current treatments in late-stage trials have been suggested to have higher efficacy the further the lesion is from the fovea [5, 33], thus extending time of foveal preservation. Therefore, there is a challenge here to accurately identify and robustly support patients at risk of foveal involvement in future whilst their visual acuity remains good, in order to maximise potential to preserve vision with these treatments.
Given the heterogeneity of GA in terms of progression, observation of recent progression over time with multi-modal retinal imaging could be a useful way to demonstrate the potential likelihood for the individual patient to benefit from these treatments. Further work is required to develop precise and robust risk stratification tools and to determine the time-difference in progression that patients will perceive as meaningful. Data from Colijn and colleagues’ analysis of four population-based cohort studies [16] suggests that delaying progression to foveal involvement by at least 0.8 years could allow the average individual with non-foveal GA to retain central vision and avoid severe vision loss for the rest of their life. [34] As such, even a modest reduction in rate of progression could deliver clinically meaningful benefits to a large number of patients.
Within the Burden construct, the increased acceptance of every other month injections is worth highlighting, particularly given recent 24-month outcome data from the DERBY and OAKS phase 3 registration trials. These trials demonstrate a marginal difference in GA growth reduction between the monthly and every other month treatment regimen (19% reduction for eyes treated monthly vs 16% reduction for eyes treated every other month in DERBY; 22% reduction for eyes treated monthly vs 18% reduction for eyes treated every other month in OAKS) [35]. On the other hand, monthly injections in these trials were associated with a near doubling of the rate of exudative choroidal neovascularisation (11.9% in monthly versus 6.7% when treated every other month). Similar rates of choroidal neovascularisation have been reported in the avacincaptad pegol trials [36]. An every-other-month regime could thus deliver increased adherence and persistence, a better safety profile (almost 50% reduction in neovascularisation risk) and greater cost-effectiveness for healthcare funders, with only a minimal reduction in efficacy.
Furthermore, participants’ fear of wet AMD risk commonly emerged as an off-putting aspect of treatment, although for some participants this was less of a concern because of the availability of a more efficacious treatment for wet AMD, or if they were already being treated for wet AMD. Even for study participants generally accepting of the GA treatment, the prospect of injections on the same day for wet AMD and for GA was burdensome (although there was one participant – P26 – who would welcome the convenience of consecutive same-day injections). A 2–3 fold increased risk of wet AMD as demonstrated in the phase 3 trials [33, 36] may necessitate regular monitoring with retinal imaging for these patients associated with increased costs to payers. Innovative patient pathways and service delivery will be required to rollout these treatments. Shared-care models involving monitoring by community optometrists may help expand capacity and reduce time spent in hospital clinics.
Listening to our study’s participants, it is vital that patients are effectively counselled on the natural history of GA and accurate expectations of treatment effects; including the fact that they are unlikely to perceive treatment benefits directly, and can expect their GA to continue to progress, albeit at a slower pace. Treatment initiation should follow a shared decision-making process involving the patient and their eye care team [37, 38]. Since participants also noted that their stance on treatment may change over time, counselling on treatment expectations will need to take place regularly to support adherence [10].
Our results confirm that longer-acting therapies which slow progression to a higher degree or halt atrophy remain an unmet need and must be the focus for future drug development. In the meantime, more frequent ocular assessment may well be welcomed by many GA patients, who are currently discharged from eye clinics in the UK, with no targeted psychosocial support for what is a progressive and debilitating disease [39, 40].
Strengths and limitations
Initially conceived as an exploratory pilot study, our study has a number of limitations. Firstly, as a relatively small-scale study involving patients from two London-based sites, there is limited generalisability to other contexts, for example other geographies in the UK (e.g., rural populations) or other countries with different eye care systems. Secondly, our system of categorisation of participants into three acceptability groups was undertaken in response to emergent patterns in our framework matrix, but did not follow a standardised method that had been predetermined in our protocol. This categorisation could variously be considered too subjective or reductive, and our forthcoming larger, multi-site quantitative study will provide a more robust, generalisable quantification of GA treatment acceptability. Thirdly, while the TFA was used to analyse the data, our interview topic guide was not systematically developed from the TFA; instead, more open-ended questions were used to explore participants’ hopes, beliefs and concerns around treatment, based on our literature review and the insights of our study’s patient advisory group. This meant that for certain TFA constructs (e.g. Ethicality and Self-efficacy), there was less rich discussion than there may have been, had the TFA been used expressly to shape the topic guide.
Nonetheless, this is the first study systematically exploring prospective acceptability of GA intravitreal therapy among a diverse sample of patients, recruited using maximum variation sampling to try to ensure participants were representative of the broader GA population. The quantitative element helps to corroborate and (tentatively) quantify interpretations made on the basis of the qualitative data; indeed, there was close alignment between responses to the Likert-type scale questions and patterns in the qualitative data. Analysis of the qualitative data using the robust Theoretical Framework of Acceptability allowed us to make sense of a rich and complex dataset, and to identify the key motivating factors driving acceptability and what most concerns GA patients and could be modified in future.
Conclusion
In summary, a majority of participants ( ~ 60%) were positive about GA treatment, despite the potential inconvenience and burdens. Participants’ key concerns related to the modest efficacy of treatment, the risk of wet AMD and side effects, and logistical issues associated with frequent, potentially lifelong treatment. We observed a sharp increase in patient acceptability when considering an every-other-month treatment regimen in comparison to monthly treatment. Given encouraging efficacy and safety outcomes for the every-other-month regimen, this may be an optimal dosing label for patients, payers and health services.
Further research in a larger population of patients with GA is required to confirm our findings, and identify any correlations between patient acceptability and structural and functional biomarkers of GA severity. We expect such research to aid patient education, selection and individualisation of treatment regimes.
Summary
What was known before
-
Intravitreal injection treatments for Geographic Atrophy (GA) are currently showing promising results in Phase 3 clinical trials, significantly slowing down (although not stopping or reversing) GA progression.
-
The acceptability of emerging treatments to patients is a vital consideration, in order to support design and delivery of interventions that patients will adhere to and persist with in the real world.
What this study adds
-
Sixty percent of participants would opt for the intravitreal treatments to slow GA progression in spite of potential treatment burdens.
-
Participants’ key concerns related to the modest efficacy of treatment, the risk of wet AMD and side effects, and logistical issues associated with frequent, potentially lifelong treatment.
-
Our study illustrated a sharp increase in patient acceptability when considering an every-other-month treatment regimen in comparison to monthly treatment.
-
Common misunderstandings regarding the workings and likely effects of the intravitreal treatments demonstrate a need for clear, accessible patient education tools.
Data availability
The raw datasets generated during and analysed during the current study are not publicly available, because the in-depth and specific information they contain could compromise the privacy of the participants, given that most participants were recruited from a single London-based site. However, elements of the anonymised raw data may be shareable on reasonable request; in which case, please contact the corresponding author for further information.
References
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96:752–6.
Dinah C, Enoch J, Ghulakhszian A, Taylor DJ, Crabb DP. Intravitreal treatment for geographic atrophy: coming soon to a patient near you? Eye. 2021;36:1121–3.
Varma R, Souied EH, Tufail A, Tschosik E, Ferrara D, Zhang J, et al. Maximum reading speed in patients with geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:195–201.
Krezel AK, Hogg R, Lohfeld L, Chakravarthy U, Azuara-Blanco A. Core outcomes for geographic atrophy trials. Br J Ophthalmol. 2020;104:1196–202.
Lally DR, Heier JS, Sadda S, Eichenbaum DA & Danzig C Progression of Atrophy in AMD: Post Hoc Analysis From the GATHER1 Study. 2022. https://investors.ivericbio.com/static-files/4341199c-b031-45a0-bc6f-a032a44a8021 [cited 2022 Sep 1].
Heier J, Singh R, Wykoff C, Steinle N, Boyer D, Monés J, et al. Efficacy of intravitreal pegcetacoplan in geographic atrophy: 24-month results from the phase 3 OAKS and DERBY trials. In 2022 [cited 2022 Nov 28]. Available from: https://investors.apellis.com/static-files/78d1b209-7324-4c4c-8b20-bf7778493bae.
Loewenstein A, Trivizki O. Future perspectives for treating patients with geographic atrophy. Graefe’s Arch Clin Exp Ophthalmol. 2022. https://doi.org/10.1007/s00417-022-05931-z.
Hutton D. Industry leaders react to FDA’s pegcetacoplan approval. Ophthalmology Times [Internet]. 2023; Available from: https://www.ophthalmologytimes.com/view/industry-leaders-react-to-fda-s-pegcetacoplan-approval.
Ehlken C, Ziemssen F, Eter N, Lanzl I, Kaymak H, Lommatzsch A, et al. Systematic review: Non-adherence and non-persistence in intravitreal treatment. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:2077–90.
Droege KM, Muether PS, Hermann MM, Caramoy A, Viebahn U, Kirchhof B, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefe’s Arch Clin Exp Ophthalmol. 2013;251:1281–4.
Finger RP, Daien V, Eldem BM, Talks JS, Korobelnik JF, Mitchell P, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration - a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20:294.
Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23:127–40.
Senra H, Ali Z, Balaskas K, Aslam T. Psychological impact of anti-VEGF treatments for wet macular degeneration: a review. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:1873–80.
Thier A, Holmberg C. The patients’ view: age-related macular degeneration and its effects–a meta-synthesis. Disabil Rehabil. 2022;44:661–71.
Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–90.
Colijn JM, Liefers B, Joachim N, Verzijden T, Meester-Smoor MA, Biarnés M, et al. Enlargement of geographic atrophy from first diagnosis to end of life. JAMA Ophthalmol. 2021;139:743–50.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: An overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88.
Klaic M, Kapp S, Hudson P, Chapman W, Denehy L, Story D, et al. Implementability of healthcare interventions: An overview of reviews and development of a conceptual framework. Implement Sci. 2022;17:1–20.
Klassen AC, Creswell J, Plano Clark VL, Smith KC, Meissner HI. Best practices in mixed methods for quality of life research. Qual Life Res. 2012;21:377–80.
Enoch J, Ghulakhszian A, Crabb DP, Dinah C, Taylor DJ. Acceptability of intravitreal injections in geographic atrophy: Protocol for a mixed-methods pilot study. BMJ Open. 2021;11:e049495.
Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized phase 2 trial. Ophthalmology. 2020;127:186–95.
Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, et al. C5 inhibitor Avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–86.
Freeman WR, Bandello F, Souied EH, Guymer RH, Garg S, Chen FK, et al. Phase 2b Study of Brimonidine DDS: Potential novel treatment for geographic atrophy. Invest Ophthalmol Vis Sci. 2019;60:971.
UK Government. Regional ethnic diversity [Internet]. 2020 [cited 2022 Sep 9]. Available from: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/regional-ethnic-diversity/latest.
Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18:179–83.
Holz FG, Ho A, Khanani AM, Chang A, Bliss C, Sharp D, et al. Efficacy of Pegcetacoplan in subgroups defined by distance from the foveal center point in the phase 3 OAKS and DERBY studies of patients with geographic atrophy. In: The Macula Society 45th Annual Meeting, Berlin, Germany. 2022. Available from: https://investors.apellis.com/static-files/adcd2696-44f6-41f2-bbb8-4cb517cdd67c [cited 2022 Sep 13].
Richardson SJ, Carroll CB, Close J, Gordon AL, O’Brien J, Quinn TJ, et al. Research with older people in a world with COVID-19: identification of current and future priorities, challenges and opportunities. Age Ageing. 2020;49:901–6.
Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Analysing qualitative data. (Routledge, London, 1994) 173–94.
Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117.
Guest G, MacQueen KM, Namey EE. Applied Thematic Analysis. (Sage, Thousand Oaks, 2012).
Künzel SH, Möller PT, Lindner M, Goerdt L, Nadal J, Schmid M, et al. Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:63.
Keenan TD, Agrón E, Domalpally A, Clemons TE, van Asten F, Wong WT, et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125:1913–28.
Goldberg R, Heier J, Wykoff CC, Staurenghi G, Singh RP, Steinle N, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. 2022. https://investors.apellis.com/static-files/ed761716-e969-4d23-a352-541fa01fd557 [cited 2022 Aug 31].
Sadda SR, Sarraf D. Therapeutic Margin for Geographic Atrophy: The Race Between Longevity and Disease Progression. JAMA Ophthalmol. 2021;139:751–2.
Singh RP, Boyer DS, Lad EG, Holz FG, Bliss C, Wong JG, et al. Efficacy of Intravitreal Pegcetacoplan in Geographic Atrophy: 24-Month Results from the Phase 3 OAKS and DERBY Trials. 2022. https://investors.apellis.com/static-files/a1ec9fdb-ef70-49c1-ae6e-5ad7caa63abe [cited 2022 Oct 24].
Iveric Bio. Iveric Bio Announces Positive Topline Data from Zimura® GATHER2 Phase 3 Clinical Trial in Geographic Atrophy [Internet]. Parsippany, New Jersey; 2022 Sep [cited 2022 Sep 9]. Available from: https://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-topline-data-zimurar-gather2-phase.
Bouaziz M, Cheng T, Minuti A, Denisova K, Barmettler A. Shared decision making in ophthalmology: A scoping review. Am J Ophthalmol. 2021;237:146–53.
Scheffer M, Menting J, Roodbeen R, van Dulmen S, van Hecke M, Schlingemann R, et al. Patients’ and health professionals’ views on shared decision‐making in age‐related macular degeneration care: A qualitative study. Ophthalmic Physiol Opt. 2022;42:1015–22.
Taylor DJ, Jones L, Binns AM, Crabb DP. ‘You’ve got dry macular degeneration, end of story’: A qualitative study into the experience of living with non-neovascular age-related macular degeneration. Eye. 2020;34:461–73.
Carlton J, Barnes S, Haywood A. Patient Perspectives in Geographic Atrophy (GA): Exploratory qualitative research to understand the impact of GA for patients and their families. Br Ir Orthopt J. 2019;15:133.
Acknowledgements
We would like to thank each of the thirty participants who took part in this study and generously volunteered their time and insights. We would also like to thank the members of our GA Patient Advisory Group for their invaluable input into developing the study.
Funding
National Institute for Health Research (NIHR) Enabling Involvement Fund (EIF) (Grant number EIFApp ID: 397). City, University of London School of Health Sciences Higher Education Innovation Fund (HEIF). Apellis Pharmaceuticals (Grant ID: AMR-000001). The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by authors JE, AG, DJT and CD. Expert support regarding treatment acceptability and the Theoretical Framework of Acceptability was provided by MS. Supervision was provided by DPC and CD. The first draft of the manuscript was written by JE and all authors commented on several draft versions of the manuscript. All authors read and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Competing interests
Jamie Enoch, Arevik Ghulakhszian and Mandeep Sekhon declare that they have no competing interests. David P Crabb reports grants from Roche, grants and personal fees from Santen, grants and personal fees from Apellis, grants from Allergan, personal fees from Thea, personal fees from Bayer and personal fees from Centervue, outside the submitted work. DPC receives funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant 116076 (Macustar). This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation program and European Federation of Pharmaceutical Industries and Associations (EFPIA). The communication reflects the author’s view and that neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein. Deanna J Taylor holds a research grant from Apellis. Christiana Dinah has served on advisory boards for Novartis, AbbVie, Ora Clinical, Roche and Apellis. CD is on the scientific advisory board for Ora Clinical, has received speaker fees from Roche and Novartis and holds a research grant from Apellis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enoch, J., Ghulakhszian, A., Sekhon, M. et al. Exploring patient acceptability of emerging intravitreal therapies for geographic atrophy: A mixed-methods study. Eye 37, 3634–3642 (2023). https://doi.org/10.1038/s41433-023-02571-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02571-3
This article is cited by
-
Piloting a forced-choice task to elicit treatment preferences in geographic atrophy
BMC Research Notes (2023)