Abstract
Background/Objectives
To investigate post-enucleation outcomes and assess the effect of extrascleral extension (ESE) on these outcomes for patients with uveal melanoma (UM) managed at a tertiary referral centre in Scotland.
Subjects/Methods
Retrospective review of all cases of UM managed by the Scottish Ocular Oncology Service for which enucleation was undertaken between 13/03/2008 and 31/12/2020. Primary outcomes were length of survival, time-to-metastasis (TTM) and local recurrence rate. Secondary outcomes were the effects of the presence of ESE, ESE size, and the use of adjuvant external beam radiotherapy (EBRT) on the primary outcomes.
Results
Of 172 enucleated UMs, 32 (18.6%) had ESE. Over a median follow-up period of 33.7 months (range = 1.1–163.7 months), 91 (52.9%) patients died. The median length of all-cause survival of 54.1 months (range = 1.1–163.7 months). One-year, 5-year, and 10-year survival rates were 84.8%, 49.1%, and 30.9%, respectively. Eighty-four (49.7%) patients had metastatic disease. The median TTM of 42.2 months (range = 0.4–106.8 months). Proportions of patients who developed metastases within 1-year, 5-years and 10-years post-enucleation were 22.7%, 52.8%, and 71.8%, respectively. There was one instance of local orbital recurrence. ESE was associated with a significantly shorter length of survival (p = 0.03). There was a trend towards a shorter length of survival and TTM with ESE > 5 mm and those who received adjuvant EBRT.
Conclusions
ESE was present in one-sixth of our cohort and was associated with a significantly shorter length of survival, particularly in the presence of ESE > 5 mm or high-risk characteristics warranting adjuvant EBRT. This data will aid prognostication of the patients in our service.
Similar content being viewed by others
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults with an incidence of ~1.3–8.6 per million [1]. It most frequently involves the choroid which comprises 85–90% of UM cases, while the iris and ciliary body are less commonly involved [2]. Studies have demonstrated all-cause survival rates for choroidal melanomas of around 80% at 5 years, around 65% at 10 years, and 47% at 15 years [3,4,5]. The rate of metastasis in UM has been shown to be around 18% at 5 years, and around 25% at 10 years [6]. The most common site of metastasis is the liver via the haematogenous route with less common sites including the skin, lung, bone and brain [7].
The management of UM has evolved towards eye- and vision-conserving therapies, including the use of plaque brachytherapy and proton beam therapy. Enucleation was historically first-line treatment for UM but the Collaborative Ocular Melanoma Study helped to shift attitudes, concluding that brachytherapy was non-inferior to enucleation for medium-sized tumours [8]. However, the threshold for enucleation is lowered in cases where there are concerns with regards to the tumour size, tumour recurrence, or extrascleral extension (ESE).
ESE is defined as tumour invasion through the corneoscleral envelope and is associated with a higher mortality rate due to its association with the development of metastatic disease [9, 10]. ESE has been reported to occur in 8–14.6% of uveal melanomas (UMs) [10,11,12,13]. The presence of ESE is not always detectable clinically, and often relies on histopathogical examination of an enucleated specimen. ESE is more likely to develop in tumours of a more advanced stage and is associated with specific microscopic histopathological tumour characteristics such as a diffuse growth pattern, an epithelioid cell-type, a higher mitotic rate, the presence of extracellular closed loops [10, 13].
The use of external beam radiotherapy (EBRT) for UM tends to be reserved for cases of local recurrence following enucleation but can be considered for cases where the risk of recurrence is judged to be high, such as a positive optic nerve resection margin on an enucleated sample. Although metastatic melanoma is considered relatively radioresistant, studies have demonstrated regression when used for small intraocular tumours. Hykin and colleagues suggested the use of EBRT to avoid radical management such as exenteration with some positive results [14]. However, another study found no benefit from the use of EBRT, rather some long-term and sometimes disfiguring side effects [15].
The primary aim of this study is to describe the length of survival, time-to-metastasis (TTM), and local recurrence rate in our cohort of patients with UM who underwent enucleation. In addition, we investigate the effect of histopathologically-proven ESE on these outcomes. We also report our experience of the use of adjuvant EBRT for ESE deemed to be at high-risk of local recurrence.
Methods
The Scottish Ocular Oncology Service (SOOS) is a national multi-disciplinary service based at Gartnavel General Hospital, Glasgow, Scotland.
This study included all patients managed by the SOOS who underwent enucleation for UM between 17th March 2008 and 31st December 2020. Iris melanomas were excluded from this study.
Data extraction
Electronic clinical notes were retrospectively reviewed to extract pre- and post-operative clinical characteristics. Histopathological data was extracted retrospectively from Pathology reports using an extraction template based on the Royal College of Pathologists dataset for reporting of UMs [16]. Guidance on Tumour-Node-Metastasis staging published by The American Joint Committee on Cancer (AJCC) was used to classify tumours [17].
Ultrasound reports were reviewed retrospectively for reported evidence of retro-scleral extension and the reported basal dimensions and height of the tumour on ultrasound imaging prior to enucleation. If ultrasound reports were unavailable, this data was extracted from pre-operative cross-sectional imaging reports if available. Surveillance liver screening imaging reports were reviewed to determine metastatic status.
Outcomes
Our primary outcomes were length of survival, TTM and local recurrence rate. The study follow-up duration was defined as the time from enucleation to either death or last healthcare encounter. A censor date of 31st October 2021 was used.
Length of survival was defined as the duration from enucleation to death, regardless of cause of death. If the patient remained alive at the end of the study period, data was censored at the date of the most recent healthcare encounter.
TTM was defined as the duration from enucleation to clinical evidence of metastasis or metastatic disease confirmed on cross-sectional imaging. Analysis of this outcome included only those patients who did not have a diagnosis of metastatic disease at the time of, or 1 week after, enucleation. Patients with metastatic disease from another primary source at time of enucleation were also excluded from analysis. If the patient did not develop metastatic disease by the end of the study period or died before developing clinical or radiological evidence of metastasis, the data were censored at the date of the most recent surveillance scan.
Local recurrence was defined as any evidence of recurrent disease within the orbit, identified either by clinical examination or radiological imaging.
Statistical analysis
The Kolmogorov–Smirnov normality test was used to assess whether datasets were parametric. For parametric data, summary statistics were described as the mean, standard deviation and range of the data. For non-parametric data, summary statistics were described as the median and range of the data.
Statistical comparisons for data between two groups were made using the unpaired Student’s t test for parametric variables and were made using the Mann–Whitney-U test for non-parametric variables.
For time-to-event outcomes, for single group analyses a Kapler–Meier survival curve was generated and the median was reported alongside the 1-year, 5-year and 10-year event rate. For comparative analyses of two groups, a Kapler–Meier survival curve was generated for each group and groups were statistically compared using the log-rank test. A p value of ≤0.05 was deemed statistically significant.
Ethics statement
It was confirmed with the local ethics committee that ethical approval was not required for this study. The study was performed in line with the Tenets of the Declaration of Helsinki.
Results
Entire study cohort
Baseline demographics
During this 13-year period, 172 ciliary body/choroidal melanomas were enucleated and of these, histopathological ESE was identified in 32 (18.6%) samples. Overall, the mean age at time of enucleation was 65.1 ± 14.2 years (range = 17.7–92.3 years) and 86 (50.0%) patients were female. There were no instances of bilateral disease. The median length of study follow-up of these patients was 33.5 months (range = 1.1–163.7 months) (Table 1).
Histopathological characteristics
Of the 172 samples, histopathological characteristics were available for 168 (97.7%) samples, the remaining 4 (2.3%) demonstrating extensive necrosis (Table 2).
The mean largest basal diameter on histopathology was 13.7 ± 4.9 mm (range = 3–24 mm) and the mean largest height was 8.6 ± 3.9 mm (range = 1–16 mm). The AJCC classification was available for 165 (96.0%) patients: 17 (10.3%) were stage I; 53 (32.1%) were stage II; 77 (46.7%) were stage III; and 18 (10.9%) were stage IV. The AJCC T-category subclassification was T1 for 22 (13.3%) samples; T2 for 32 (19.3%) samples; T3 for 65 (39.2%) samples; T4 and 47 (28.3%) samples.
Post-operative characteristics
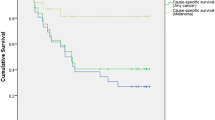
Within our follow-up period, 91 (52.9%) patients died of which the cause of death was available for 60 (67.0%) patients. Of these, the primary cause of death was either confirmed to be or likely to be metastatic melanoma for 56 (93.3%) patients. The median length of survival post-enucleation was 54.1 months (range = 1.1–163.7 months) (Fig. 1). The 1-year, 5-year, and 10-year survival rates post-enucleation were 84.8%, 49.1%, and 30.9% respectively.
Three patients had known metastatic disease from other primary sources prior to enucleation for primary choroidal melanoma and therefore were excluded from further analysis of TTM. Within our follow-up period, of the remaining 169 patients, 84 (49.7%) patients had associated metastatic disease: 7 (4.1%) had metastatic disease prior to enucleation, 8 (4.7%) were diagnosed with metastatic disease in the week following enucleation, and 69 (40.8%) developed metastatic disease post-enucleation.
Excluding those who were found to have metastatic disease prior to enucleation and within 1 week of enucleation, the median TTM was 42.2 months (range = 0.4–106.8 months) (Fig. 2) for those with available screening imaging (n = 145). The proportions of these patients who had developed metastases within 1 year, 5 years and 10 years post-enucleation were 22.7%, 52.8%, and 71.8% respectively.
a Kaplan–Meier plot comparing length of survival (days) between primary enucleation cohort and secondary enucleation cohort; b Kaplan–Meier plot comparing time-to-metastasis (days) between primary enucleation cohort and secondary enucleation cohort; c Kaplan–Meier plot comparing length of survival (days) for those who underwent secondary enucleation due to recurrence of disease and those who underwent secondary enucleation for other indications. Median length of survival was 74.9 months for the former group and not calculable in the latter group due to an insufficient event rate; d Kaplan–Meier plot comparing time-to-metastasis (days) for those who underwent secondary enucleation due to recurrence of disease and those who underwent secondary enucleation for other indications. Median length of survival was 64.2 months for the former group and not calculable in the latter group due to an insufficient event rate.
During our follow-up period, there was one instance of local recurrence within the orbit following secondary enucleation in a patient with ESE without distant metastases and stage 3 disease. Following histological confirmation of tumour recurrence, the patient underwent exenteration with adjuvant EBRT. The patient has remained metastasis-free.
Primary enucleation compared to secondary enucleation
Of 172 enucleations, 75 (43.6%) were primary enucleations and 97 (56.4%) were secondary enucleations. Of the latter, 32 (33.0%) patients previously underwent plaque therapy, 46 (47.4%) patients previously underwent proton beam therapy, and the remaining 19 (19.6%) patients previously underwent a sequential combination of eye-conserving therapies. The indications for secondary enucleation were recurrence of intraocular disease for 59 (60.8%) patients, an intractably painful eye for 24 (24.7%) patients, clinical/radiological suspicion of ESE for 3 (3.1%) patients, and miscellaneous reasons for the remaining 11 (11.3%) patients.
The mean age of patients who underwent primary enucleation was 68.5 ± 14.2 years compared to 62.6 ± 13.7 years for patients who underwent secondary enucleation (p = 0.02). Comparing primary enucleations versus secondary enucleations using the AJCC classification, 4.1% versus 15.4% respectively were stage I; 20.3% versus 42.9% respectively were stage II; 70.3% versus 31.9% respectively were stage III; and 5.4% versus 8.8% respectively were stage IV.
The median length of survival following primary enucleation was 34.0 months (range = 1.1–139.4 months) compared to 85.7 months (range = 3.1–163.7 months) following secondary enucleation (p = 0.005).
The median TTM post-enucleation following primary enucleation was 19.9 months (range = 0.4–123.9 months) compared to 83.0 months (range = 0.7–149.8 months) following secondary enucleation (p = 0.003).
Sub-group analysis comparing those who underwent secondary enucleation due to disease recurrence and those who underwent secondary enucleation for other indications demonstrated a trend towards a shorter length of survival (p = 0.13) and a significantly shorter TTM (p = 0.02) for the former group (Fig. 2).
Cohort with ESE
Pre-operative characteristics
In the population of patients with ESE (n = 32), the mean age at time of enucleation was 68.3 ± 14.9 years (range = 17.7–91.0 years) and 15 (46.9%) were female. The median length of study follow-up of these patients was 28.1 months (range = 2.2–162.7 months).
Considering the available data, ESE was evident pre-operatively at the slit lamp in 8 of 16 (50.0%) cases and pre-operatively on imaging in 9 of 27 (33.3%) cases. The location of ESE was anterior to the equator in 11 (34.4%) samples and posterior to the equator in 21 (65.6%) samples. Of the 32 samples, ESE was visible macroscopically in 27 (84.4%) specimens and present only microscopically in 5 (15.6%) specimens, four of which were posterior to the equator. The size of ESE was ≤5 mm in diameter for 26 (81.3%) samples and >5 mm in diameter for 6 (18.8%) samples. Other histopathological characteristics are shown in Table 2.
Post-operative characteristics
Within our follow-up period, 21 of 32 (65.6%) patients died of which the cause of death was available for 14 (66.7%) patients. Of these 14 patients, the primary cause of death was confirmed to be or likely to be metastatic melanoma for 12 (85.7%) patients. The median length of survival post-enucleation was 33.5 months (range = 2.2–162.7 months) in the ESE cohort, compared to a median length of survival of 63.6 months (range = 1.1–163.7 months) in the non-ESE cohort (p = 0.03) (Fig. 3).
One patient had known metastatic disease from another primary source prior to enucleation for primary choroidal melanoma and therefore was excluded from further analysis of TTM. Within our follow-up period, 20 of 31 (64.5%) patients with ESE had associated metastatic disease: 3 (9.7%) had metastatic disease at time of enucleation, 3 (9.7%) were diagnosed with metastatic disease 1 week following enucleation, and 14 (45.2%) developed metastatic disease post-enucleation. Excluding those who were found to have metastatic disease prior to enucleation and within 1 week of enucleation, for those with available screening imaging the median TTM was 31.5 months (range = 0.6–78.2 months) in the ESE cohort, compared to a median TTM of 45.1 months (range = 0.4–149.8 months) in the non-ESE cohort (p = 0.49) (Fig. 3).
ESE ≤5 mm compared to ESE >5 mm
Of the 32 patients with ESE, the size of ESE was >5 mm in 6 (18.8%) samples and ≤5 mm in 26 (81.3%) samples. The median length of survival was 25.6 months in those with ESE > 5 mm and 33.5 months in those with ESE ≤ 5 mm. The median TTM was 18.5 months in those with ESE > 5 mm and 33.1 months in those with ESE ≤ 5 mm.
Adjuvant EBRT compared to no adjuvant treatment
Of the 32 patients with ESE, 7 (21.9%) received adjuvant EBRT. One additional patient was offered adjuvant EBRT but declined. The indications for adjuvant radiotherapy included positive surgical resection margins (n = 2), orbital tumour deposits (n = 1), multiple high-risk features (n = 2), and large ESE (n = 2). Dosing regimens included 65 Gy over 30 fractions for (n = 3) patients, 55 Gy over 20 fractions (n = 3), and 56 Gy over 33 fractions (n = 1).
The median length of survival was 33.5 months in the adjuvantly-treated group and 34.6 months in the non-adjuvantly-treated group. The median TTM was 18.5 months in the adjuvantly-treated group and 73.3 months in the non-adjuvantly-treated group.
One of the seven patients who received adjuvant EBRT developed severe socket and fornix contracture requiring surgical intervention.
Discussion
Over a median follow-up period of 33.7 months, 52.9% of our cohort died and of those with a known cause of death, 93% died of metastatic UM. The median length of survival was 54.1 months and the 1-year, 5-year and 10-year survival rates were 84.8%, 49.1% and 30.9%, respectively.
Around two-thirds of our cohort with ESE died over a median follow-up period of 28.1 months and the length of survival was significantly shorter compared to those without ESE, mirroring similar evidence in the literature [10]. In addition, the trend towards a shorter length of survival and TTM in those with ESE > 5 mm is in keeping the higher T-subclassification granted to these tumours by the AJCC [17].
The underlying reasons for a shorter length of survival in the ESE cohort are likely multi-factorial and may relate to more advanced disease in those with ESE at time of enucleation. This is reflected in our cohort by the higher proportion of AJCC stage 3 and stage 4 in the ESE cohort compared to the non-ESE cohort. Inherent tumour characteristics likely also play a role as the development of ESE may reflect particularly aggressive tumour characteristics. In our ESE cohort, there was a trend towards a higher proportion of samples with a diffuse growth pattern, mixed or epithelioid cell type, a higher mitotic count, and presence of extracellular matrix patterns, all of which are associated with worse prognosis [16].
Our results also demonstrated that 49.7% of our cohort had associated metastatic disease during our follow-up period with 46.7% of the non-ESE cohort being affected, compared with 64.5% of the ESE cohort. Metastatic disease was present at or around the time of enucleation in 8.8% of our entire cohort and in 19.4% of patients with ESE, strengthening the case for multidisciplinary input at all stages so that appropriate management for metastatic disease can be instituted in a timely manner.
For those patients without metastatic disease at or around the time of enucleation, the median TTM was 42.2 months and did not statistically differ in those who had ESE compared with those who did not, though there did appear to be a trend towards a shorter TTM in the ESE group. The incidence of metastatic disease has been reported to be influenced by histological factors, increasing tumour size, ciliary body location, and genetic composition [10]. There have been few studies which have investigated the mechanism by which ESE may lead to an increased propensity for metastasis. In a healthy eye, lymphatic channels have only been shown to be present in the conjunctiva [18]. There is some evidence that the development of ESE may either allow tumour invasion into existing lymphatic channels or promote lymphangiogenesis, providing a route for metastasis [19].
Of note, 18.6% of our cohort demonstrated histopathological evidence of ESE. This proportion is higher than some other series of enucleation samples reported in literature [10,11,12,13, 20], though it should be noted that our cohort included secondary enucleations. A recently published case series with a similar sample size to our study has described a comparable rate of extraocular extension of 19.4% [21].
In our cohort, ESE was more likely to be found posterior to the equator and to be <5 mm in diameter, with a small proportion of samples having only microscopic ESE detected on histopathological assessment. It can also be seen that diagnosis of ESE pre-enucleation can be challenging due to its posterior location and relatively low detection rates on clinical and radiological assessment. Ultrasound assessment is also impeded due to the enhanced depth of imaging required leading to attenuation and shadowing effects. The challenges in detecting posterior ESE clinically and radiologically have also been described by Burris et al. [20].
Orbital recurrence following enucleation have been reported in the literature in up to 18% of UM with ESE [10, 22, 23]. Adjuvant EBRT post-enucleation has been proposed to reduce the risk of local recurrence and subsequent incidence of metastatic disease. Its use to prevent local recurrence is a matter of debate and clear evidence to guide clinicians on which patients benefit most from this therapy does not currently exist. In our series, this treatment was offered to those with ESE determined through multidisciplinary discussion to be at highest risk of local recurrence and following a fully informed discussion with the patient. The data in our series is understandably small though there was a trend towards a shorter length of survival and shorter TTM in those patients for whom adjuvant EBRT was offered, likely due to the co-existence of other poor prognostic factors. However, in our cohort there was only one instance of local recurrence, suggesting that current treatment modalities are successful in minimising the rate of local recurrence.
Few studies have investigated the use of adjuvant EBRT for UM [14, 15]. One analysis included 51 patients with histopathological evidence of ESE post-enucleation, of which 22 had EBRT. The authors found no cases of local recurrence and no difference in survival rates within the 23-year study period. A high rate of radiotherapy side effects including socket contracture, persistent inflammation and discomfort, and implant exposure were noted [15]. Of the 7 patients in our cohort who received EBRT, one suffered severe socket and fornix contracture.
Limitations of this study
This study describes a cohort of patients who primarily reside in Scotland and were managed at a single tertiary centre. Therefore, the findings of this study are not directly comparable to other populations where population demographics, offered treatments, and treatment thresholds may differ.
In addition, the samples sizes within groups for some analyses are relatively small. Therefore, particularly for sub-group analyses of the ESE cohort, the analyses may not be powered to detect a statistically significant difference if one does truly exist.
It is also important to note that this study included both primary and secondary enucleations and therefore the lead-time from diagnosis to enucleation and subsequent study endpoints is likely to be heterogeneous. Certainly, comparative analyses between these two groups demonstrated that those that underwent primary enucleation were more likely to be older at time of enucleation, have disease of a more advanced AJCC stage at time of enucleation, have a shorter post-enucleation length of survival, and have a shorter post-enucleation TTM than those that underwent secondary enucleation. As expected, clinical heterogeneity in the secondary enucleation cohort was also present with poorer clinical outcomes in those undergoing enucleation following recurrence of intraocular disease compared to other indications for secondary enucleation. Recurrence as a negative prognostic indicator has been previously demonstrated in the literature [24, 25] and similar findings to ours, specifically in relation to cohorts undergoing secondary enucleation, have also been described [26].
Conclusions
In our retrospective cohort study of patients with UM who underwent enucleation and were managed at a tertiary service in Scotland, over a median follow-up period of 33.7 months the median length of survival was 54.1 months. For those without a diagnosis of metastatic disease at or around the time of enucleation, the median TTM was 42.2 months. Those patients who underwent primary enucleation, compared to secondary enucleation, were more likely to have poorer prognostic indicators at the time of enucleation and had a significantly shorter length of survival and TTM. Around one-sixth of patients had ESE and this was more likely to be located posterior to the equator with a diameter of <5 mm, making clinical or radiological detection challenging prior to enucleation. The presence of ESE was associated with a significantly shortened length of survival and there was also a trend towards a shorter TTM. Adjuvant EBRT was offered to a small, selected subset of patients with ESE but the length of survival and TTM remained poorer than in those patients for whom it was not offered, likely because of the effects of high-risk features on prognosis. There was only one instance of local recurrence over the study follow-up period. This data provides useful prognostic information for clinicians managing patients with UM following enucleation as well as patients who have undergone this treatment modality.
Summary
What was known before
-
Uveal melanoma has been shown to have an all-cause survival rate of 47% at 15 years and a 10-year rate of developing metastatic disease of 15%.
-
In addition, there is no consensus on which patients should be offered adjuvant EBRT post-enucleation to reduce the risk of local recurrence.
What this study adds
-
The data from this study will aid clinicians in imparting important prognostic information for patients who have underwent enucleation for UM.
-
Extrascleral extension, particularly if more than 5 mm in size, is associated with a significantly shorter length of survival post-enucleation.
Data availability
Anonymised datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, BiggeriZ A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology 2007;114:2309–15.
McLaughlin CC, Wu X-C, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer [Internet] 2005;103:1000–7. https://doi.org/10.1002/cncr.20866.
Beasley AB, Preen DB, McLenachan S, Gray ES, Chen FK. Incidence and mortality of uveal melanoma in Australia (1982–2014). Br J Ophthalmol. 2021. https://doi.org/10.1136/bjophthalmol-2021-319700.
Jamison A, Bhatti LA, Sobti MM, Chadha V, Cauchi P, Kemp EG. Uveal melanoma-associated survival in Scotland. Eye (Lond). 2019;33:1699–706.
Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006;124:1684–93.
Shields C, Dockery P, Mayro E, Bas Z, Yaghy A, Lally S, et al. Conditional survival of uveal melanoma using The Cancer Genome Atlas (TCGA) classification (Simplified Version) in 1001 cases. Saudi J Ophthalmol. 2022. https://doi.org/10.4103/SJOPT.SJOPT_69_21.
Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye [Internet] 2017;31:241–57. http://www.nature.com/articles/eye2016275.
Diener-West M, Earle J, Fine S, Hawkins B, Moy C, Reynolds S, et al. Collaborative Ocular Melanoma Study Group: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III. Arch Ophthalmol. 2001;119:969–82.
Li W, Gragoudas ES, Egan KM. Metastatic melanoma death rates by anatomic site after proton beam irradiation for uveal melanoma. Arch Ophthalmol (Chic, Ill 1960) [Internet] 2000;118:1066–70. http://www.ncbi.nlm.nih.gov/pubmed/10922199.
van Beek JGM, Koopmans AE, Vaarwater J, de Rooi JJ, Paridaens D, Naus NC, et al. The Prognostic Value of Extraocular Extension in Relation to Monosomy 3 and Gain of Chromosome 8q in Uveal Melanoma. Investig Opthalmology Vis Sci [Internet] 2014;55:1284 http://iovs.arvojournals.org/article.aspx?
Kujala E, Makitie T, Kivela T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig Opthalmology Vis Sci [Internet] 2003;44:4651 http://iovs.arvojournals.org/article.aspx?
Singh A, Shields C, Shields J. Prognostic factors in uveal melanoma. Clin Ophthalmic Oncol Uveal Tumors. 2014;11:239–48.
Coupland SE, Campbell I, Damato B. Routes of Extraocular Extension of Uveal Melanoma. Risk Factors and Influence on Survival Probability. Ophthalmology 2008;115:1778–85.
Hykin PG, Mccartney ACE, Plowman PN, Hungerford JL. Postenucleation orbital radiotherapy for the treatment of malignant melanoma of the choroid with extrascleral extension [Internet]. 74, Br J Ophthalmol. 1990 [cited 2020 Oct 9]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1041975/pdf/brjopthal00575-0044.pdf.
Roelofs KA, Cohen VML, Sagoo MS, Plowman PN, Negretti GS, O’Day R, et al. Adjuvant External Beam Radiotherapy Following Enucleation of Eyes With Extraocular Extension From Uveal Melanoma. Ophthalmic Plast Reconstr Surg [Internet]. 2020 [cited 2020 Oct 16];Publish Ah. https://journals.lww.com/10.1097/IOP.0000000000001800
Mudhar HS, Coupland SE. Dataset for histopathological reporting of uveal melanoma. 5th ed [Internet]. 2021. Available from: https://www.rcpath.org/uploads/assets/7baa5a48-e3fc-4dc8-b0e36c5a5c9a194b/778f7dc3-135a-4590-aaeec103048a492f/G056-Dataset-for-histopathological-reporting-of-uveal-melanoma.pdf.
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. editors. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2016. p. 805–17.
Cursiefen C, Kruse FE, Naumann GOH. Cornea and limbus. Appl Pathol Ophthalmic Microsurg. Berlin: Springer; 2008. p. 97–130.
Heindl LM, Hofmann TN, Adler W, Knorr HLJ, Holbach LM, Naumann GOH, et al. Intraocular tumor-associated lymphangiogenesis a novel prognostic factor for ciliary body melanomas with extraocular extension? Ophthalmology 2010;117:334–42. https://doi.org/10.1016/j.ophtha.2009.06.057.
Burris CKH, Papastefanou VP, Thaung C, Restori M, Arora AK, Sagoo M, et al. Detection of Extrascleral Extension in Uveal melanoma with Histopathological Correlation. Orbit 2018;37:287–92. https://doi.org/10.1080/01676830.2017.1423083.
Negretti GS, Gurudas S, Gallo B, Damato B, Arora AK, Sivaprasad S, et al. Survival analysis following enucleation for uveal melanoma. Eye. 2022;36:1669–74.
Bergeron E, Lihimdi N, Bergeron D, Landreville S. Orbital recurrence of iris melanoma 21 years after enucleation. BMJ Case Rep [Internet]. 2017 [cited 2021 Dec 14];2017. http://www.ncbi.nlm.nih.gov/pubmed/28882848.
Mittica N, Vemuganti GK, Duffy M, Torczynski E, Edward DP. Late orbital recurrence of a choroidal melanoma following internal resection: Report of a case and review of the literature. Surv Ophthalmol. 2003;48:181–90.
Gallie BL, Simpson ER, Saakyan S, Amiryan A, Valskiy V, Finger PT, et al. Local Recurrence Significantly Increases the Risk of Metastatic Uveal Melanoma. Ophthalmology 2016;123:86–91.
Jampol LM, Moy CS, Murray TG, Reynolds SM, Albert DM, Schachat AP, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS Rep. no 19 Ophthalmol 2002;109:2197–206.
Wang H, Zhang R, Wang Y, Chen R, Liu Y, Li Y, et al. Retrospective analysis of secondary enucleation for uveal melanoma after plaque radiotherapy. BMC Ophthalmol. 2022;22:163.
Author information
Authors and Affiliations
Contributions
Conceptualisation of study aims: VC; Data acquisition: FR, DS, TM; Data extraction, statistical analysis, data interpretation, preparation of paper: DS, TM; Critical review of paper: FR, JC, PC, VC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarode, D., McClay, T., Roberts, F. et al. Post-enucleation outcomes of patients with uveal melanoma in Scotland. Eye 37, 988–994 (2023). https://doi.org/10.1038/s41433-022-02280-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02280-3