Abstract
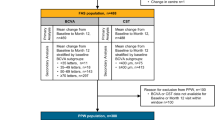
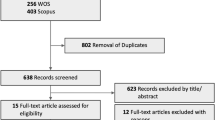
This systematic review and meta-analysis investigated the impact of anti-vascular endothelial growth factor (VEGF) treatment in management of eyes with non-proliferative diabetic retinopathy (NPDR) without centre involving diabetic macular oedema (CI-DMO). We searched multiple databases for all randomised clinical trials (RCTs) that evaluated anti-VEGF treatment versus observation in eyes with NPDR without CI-DMO. Data was collected for six outcomes (best corrected visual acuity (BCVA) improvement, diabetic retinopathy severity score (DRSS), central subfield thickness, progression to vision threatening complications (VTCs), ocular adverse events and quality of life measures). Risk of bias was assessed using Cochrane risk-of-bias tool for randomised trials (RoB 2) and certainty of evidence was assessed using Grade of Recommendations, Assessment, Development and Evaluation (GRADE). We identified a total of 2 unique RCTs that compared aflibercept and sham to treat a total of 811 eyes. For BCVA change, there was a small, clinically insignificant benefit for aflibercept treatment at year 2 (MD 0.70, 95% CI 0.02–1.38, GRADE rating: MODERATE). DRSS demonstrated a statistically significant improvement with aflibercept use at year 2 (RR 3.76, 95% CI 2.75–5.13, GRADE rating: MODERATE). VTCs were significantly less in aflibercept arm at year 2 (RR 0.30, 95% CI 0.23–0.40, GRADE rating: MODERATE). In conclusion, aflibercept treatment versus observation in eyes with NPDR without CI-DMO can result in reduced risk of development of VTCs and regression of DRSS score over 2 years. Future trials are needed to increase the precision of the treatment effect and to provide data on quality-of-life metrics.
PROSPERO Registration: CRD42021288608.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do D V, Midena E, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376–85. https://pubmed-ncbi-nlm-nih-gov.login.ezproxy.library.ualberta.ca/27651226/. Accessed 27 Dec 2021.
Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet (London, England). 2017;389:2193–203. https://pubmed-ncbi-nlm-nih-gov.login.ezproxy.library.ualberta.ca/28494920/. Accessed 27 Dec 2021.
Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137. https://doi.org/10.1001/jama.2015.15217. Accessed 27 Dec 2021.
Sun JK, Glassman AR, Beaulieu WT, Stockdale CR, Bressler NM, Flaxel C, et al. Rationale and application of the protocol s anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126:87. https://doi.org/10.1016/j.ophtha.2018.08.001. Accessed 27 Dec 2021.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222. https://www-karger-com.login.ezproxy.library.ualberta.ca/Article/FullText/458539. Accessed 27 Dec 2021.
Anon. Diabetic Retinopathy PPP 2019—American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed 27 Dec 2021.
Ehlers JP, Yeh S, Maguire MG, Smith JR, Mruthyunjaya P, Jain N, et al. Intravitreal pharmacotherapies for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2022;129:88–99.
Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–55. https://pubmed-ncbi-nlm-nih-gov.login.ezproxy.library.ualberta.ca/21035872/. Accessed 2 Jan 2022.
Gupta P, Aravindhan A, Gan ATL, Man REK, Fenwick EK, Mitchell P, et al. Association between the severity of diabetic retinopathy and falls in an Asian population with diabetes: The Singapore Epidemiology of Eye Diseases Study. JAMA Ophthalmology. 2017;135:1410. https://doi.org/10.1001/jamaophthalmol.2017.4983. Accessed 28 Oct 2021.
Anon. ICTRP Search Portal. https://trialsearch.who.int/?TrialID=EUCTR2007-006795-10-GB. Accessed 19 Oct 2021.
Lim JI. Prevention of severe nonproliferative diabetic retinopathy progression with more at stake than visual acuity. JAMA Ophthalmol. 2021;139:714–6. https://jamanetwork-com.login.ezproxy.library.ualberta.ca/journals/jamaophthalmology/fullarticle/2778075. Accessed 27 Oct 2021.
Tinetti ME, Powell L. Fear of falling and low self-efficacy: a case of dependence in elderly persons. Journal of gerontology. 1993;48:35–8. https://pubmed.ncbi.nlm.nih.gov/8409238/. Accessed 28 Oct 2021.
Anon. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98:823–33. http://www.aaojournal.org/article/S0161642013380142/fulltext. Accessed 27 Oct 2021.
Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–8. https://care.diabetesjournals.org/content/40/3/412. Accessed 27 Oct 2021.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;1608–22. https://doi.org/10.1016/j.ophtha.2018.04.007. Accessed 27 Oct 2021.
Nanegrungsunk O, Bressler NM. Prevention of vision-threatening complications in diabetic retinopathy: two perspectives based on results from the DRCR Retina Network Protocol W and the Regeneron-sponsored PANORAMA. Curr Opin Ophthalmol. 2021;32. https://pubmed.ncbi.nlm.nih.gov/34419979/. Accessed 27 Oct 2021.
Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136:1138–48. https://pubmed.ncbi.nlm.nih.gov/30043039/. Accessed 27 Oct 2021.
Mitchell P, McAllister I, Larsen M, Staurenghi G, Korobelnik JF, Boyer DS, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmology. Retina. 2018;2:988–96. https://pubmed.ncbi.nlm.nih.gov/31047501/. Accessed 27 Oct 2021.
Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. ophthalmology. Retina. 2018;2:997–1009. https://pubmed.ncbi.nlm.nih.gov/31047503/. Accessed 27 Oct 2021.
Bressler SB, Liu D, Glassman AR, Blodi BA, Castellarin AA, Jampol LM, et al. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol. 2017;135:558–68. https://pubmed.ncbi.nlm.nih.gov/28448655/. Accessed 27 Oct 2021.
Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193–203. http://www.thelancet.com/article/S0140673617311935/fulltext. Accessed 27 Oct 2021.
Alagorie AR, Velaga S, Nittala MG, Yu HJ, Wykoff CC, Sadda SR. Effect of aflibercept on diabetic retinopathy severity and visual function in the RECOVERY study for proliferative diabetic retinopathy. Ophthalmol Retin. 2021;5:409–19.
Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–55. https://pubmed.ncbi.nlm.nih.gov/34351414/. Accessed 19 Oct 2021.
Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139:E1–2. https://pubmed.ncbi.nlm.nih.gov/33784735/. Accessed 19 Oct 2021.
Anon. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified airlie house classification: ETDRS report number 10. Ophthalmology. 2020;127:S99–119.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Anon. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials | Cochrane Bias. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. Accessed 19 Oct 2021.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. https://pubmed.ncbi.nlm.nih.gov/21185693/. Accessed 19 Oct 2021.
Anon. 9.5.2 Identifying and measuring heterogeneity. https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm. Accessed 22 Dec 2021.
Anon. Long-term efficacy and safety of intravitreal aflibercept injections for the treatment of diabetic retinopathy for subjects who completed the 2-year PANORAMA trial—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04708145. Accessed 19 Oct 2021.
Anon. Intravitreal bevacizumab for nonproliferative diabetic retinopathy—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04511715. Accessed 19 Oct 2021.
Anon. A multicenter, randomized study in participants with diabetic retinopathy without center-involved diabetic macular edema to evaluate the efficacy, safety, and pharmacokinetics of ranibizumab delivered via the port delivery system relative to the comparator Arm—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04503551. Accessed 19 Oct 2021.
Anon. China Clinical Trial Registration Center-Level 1 Registration Body of the World Health Organization International Clinical Trial Registration Platform. https://www.chictr.org.cn/showproj.aspx?proj=16494. Accessed 19 Oct 2021.
Anon. Multicenter Clinical Study of Anti-VEGF Treatment on High Risk Diabetic Retinopathy (DR)—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03452657. Accessed 19 Oct 2021.
Anon. ICTRP Search Portal. https://trialsearch.who.int/?TrialID=EUCTR2011-003304-20-GB. Accessed 19 Oct 2021.
Anon. Evaluation of the retina in patients with non-proliferative diabetic retinopathy after aflibercept injection in the eye—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04702048. Accessed 19 Oct 2021.
Ip MS, Zhang J, Ehrlich JS. The clinical importance of changes in diabetic retinopathy severity score. Ophthalmology. 2017;124:596–603. https://pubmed.ncbi.nlm.nih.gov/28284785/. Accessed 14 Nov 2021.
Funding
This study represents original work never presented previously.
Author information
Authors and Affiliations
Contributions
VC was responsible for study conception, design, interpretation of results and draft paper preparation. GSS was responsible for design, data collection, analysis and interpretation of results and draft paper preparation. DP, JX, MF were responsible for data collection. MRP, DZ were responsible for analysis and interpretation of results. LT, AL, FGH, SJG, PKK, MB, RHG, SFB, SS, CCW were responsible for interpretation of results, critical review and feedback on paper. All authors reviewed the results and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
VC: Advisory board: Alcon, Roche, Bayer, Novartis; Grants: Bayer, Novartis. GSS: Nothing to disclose. MRP: Nothing to disclose. DP: Nothing to disclose. JX: Nothing to disclose. DZ: Nothing to disclose. MF: Nothing to disclose. LT: Nothing to disclose. AL: Consultant: Allergan, Bayer, BeyeOnics, ForSight Labs, NotalVision, Novartis, Roche. FGH: Research funds: Acucela, Allergan, Apellis, Bayer, Bioeq/Formycon, Roche/Genentech, Geuder, Heidelberg Engineering, IvericBio, Kanghong, Novartis, NightStarX, Zeiss; Personal fees: Boehringer-Ingelheim, Grayburg Vision, LinBioscience, Pixium Vision, Stealth BioTherapeutics, Aerie, Oxurion. SJG: Consultant: Allergan, Apellis, Bausch and Lomb, Boehringer Ingelheim, Johnson and Johnson, Kanaph; Research funds: American Academy of Ophthalmology, Apellis, Boehringer Ingelheim, NGM Bio, Regeneron. RHG: Advisory boards: Bayer, Novartis, Apellis, Roche, Genentech Inc. PKK: Consultant: Novartis, Bayer, Biogen, Regeneron, Kanghong, Allergan, RegenxBio. MB: Research funds: Pendopharm, Bioventus, Acumed. SF-B: Consultant: Allergan, Bayer, Novartis. SS: Research funds: Novartis, Bayer, Allergan, Roche, Boehringer, Ingelheim and Optos Plc; Travel grants: Novartis, Bayer; Speaker fees: Novartis, Bayer and Optos Plc.; Advisory board: Novartis, Bayer, Allergan, Roche, Boehringer, Ingelheim, Optos Plz, Oxurion, Ophthea, Apellis, Oculis, Heidelberg Engineering. CCW: Consultant: Acuela, Adverum Biotechnologies, Inc, Aerpio, Alimera Sciences, Allegro Ophthalmics, LLC, Allergan, Apellis Pharmaceuticals, Bayer AG, Chengdu Kanghong Pharmaceuticals Group Co, Ltd, Clearside Biomedical, DORC (Dutch Ophthalmic Research Centre), EyePoint Pharmaceuticals, Gentech/Roche, GyroscopeTx, IVERIC bio, Kodiak Sciences Inc, Novartis AG, ONL Therapeutics, Oxurion NV, PolyPhotonix, Recens Medical, Regeron Pharmaceuticals, Inc, REGENXBIO Inc, Santen Pharmaceutical Co, Ltd, and Takeda Pharmaceutical Company Limited; Research funds: Adverum Biotechnologies, Inc, Aerie Pharmaceuticals, Inc, Aerpio, Alimera Sciences, Allergan, Apellis Pharmaceuticals, Chengdu Kanghong Pharmaceutical Group Co, Ltd, Clearside Biomedical, Gemini Therapeutics, Genentech/Roche, Graybug Vision, Inc, GyroscopeTx, Ionis Pharmaceuticals, IVERIC bio, Kodiak Sciences Inc, Neurotech LLC, Novartis AG, Opthea, Outlook Therapeutics, Inc, Recens Medical, Regeneron Pharmaceuticals, Inc, REGENXBIO Inc, Samsung Pharm Co, Ltd, Santen Pharmaceutical Co, Ltd, Xbrane Biopharma AB. No other acknowledgements to list.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhary, V., Sarohia, G.S., Phillips, M.R. et al. Role of anti-vascular endothelial growth factor in the management of non-proliferative diabetic retinopathy without centre-involving diabetic macular oedema: a meta-analysis of trials. Eye 37, 1966–1974 (2023). https://doi.org/10.1038/s41433-022-02269-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02269-y