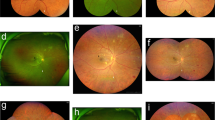
Abstract
Purpose
To validate the potential application of THEIA™ as clinical decision making assistant in a national screening program.
Methods
A total of 900 patients were recruited from either an urban large eye hospital, or a semi-rural optometrist led screening provider, as they were attending their appointment as part of New Zealand Diabetic Eye Screening Programme. The de-identified images were independently graded by three senior specialists, and final results were aggregated using New Zealand grading scheme, which was then converted to referable/non-referable and Healthy/mild/more than mild/sight threatening categories.
Results
THEIA™ managed to grade all images obtained during the study. Comparing the adjudicated images from the specialist grading team, “ground truth”, with the grading by the AI platform in detecting “sight threatening” disease, at the patient level THEIA™ achieved 100% imageability, 100% [98.49–100.00%] sensitivity and [97.02–99.16%] specificity, and negative predictive value of 100%. In other words, THEIA™ did not miss any patients with “more than mild” or “sight threatening” disease. The level of agreement between the clinicians and the aggregated results was (k value: 0.9881, 0.9557, and 0.9175), and the level of agreement between THEIA™ and the aggregated labels was (k value: 0.9515).
Conclusion
This multi-centre prospective trial showed that THEIA™ did not miss referable disease when screening for diabetic retinopathy and maculopathy. It also had a very high level of granularity in reporting the disease level. As THEIA™ has been tested on a variety of cameras, operating in a range of clinics (rural/urban, ophthalmologist-led\optometrist-led), we believe that it will be a suitable addition to a public diabetic screening program.
Similar content being viewed by others
Introduction
Implementation of artificial intelligence (AI) in medicine and particularly in ophthalmology has a long history, but also accelerating rapidly in the past few years [1,2,3,4]. So far, the most promising application of AI in ophthalmology is as a screening tool for Diabetic Retinopathy (DR) [5,6,7,8,9].
It is now well accepted that a comprehensive DR screening program can reduce the burden of diabetes related vision loss [4, 10, 11]. However, delivering large community-based programs can be a major challenge even in developed countries, such as including New Zealand which has both a high prevalence of diabetes [12] and a significant proportion of the population not being screened regularly [13]. AI based algorithms, that can reliably detect DR in retinal images and provide instantaneous reporting with high diagnostic accuracy, could significantly improve the earlier detection of DR. In addition, by enabling specialist-level diagnostics to be provided to multiple peripheral sites simultaneously these algorithms also have the potential to significantly increase access to, and lower the cost of, screening for DR [14, 15].
In recent years there have been significant advances in development of AI algorithms to assist with diabetic eye screening programs [1]. While the accuracy of AI-based models for detecting DR have been demonstrated in many previous studies [5,6,7,8,9], most have failed to perform in the “real world” setting [16]. It has been shown that most research AIs for detection of retinopathy are not generalizable, as training datasets used are not representative of the wider society, obtained from relatively homogenous populations, limited in numbers or highly curated by clinicians, contain just one image per eye, and very limited grade granularity (i.e. binary outcome for referable disease) [17].
Toku Eyes® in partnership with the Auckland and Counties Manukau District Health Boards (DHB) in New Zealand developed THEIA™, a AI DR Screening tool that is: trained and tested locally, is clinic/clinician/camera agnostic, gender/age/ethnicity unbiased, and provides retinopathy and maculopathy grading to the New Zealand Ministry of Health requirements [18]. The preliminary results of the first iteration of THEIA™, a trained and tested on a large dataset that represents 25% of New Zealanders living with diabetes, demonstrated a sensitivity of [94–95%] and specificity of [61–63%] for sight threatening DR [5]. The algorithm has subsequently been improved and optimized by a process of continuous retraining and testing. In this paper, the results of the latest iteration of THEIA™ tested in a prospective multi-centre prospective trial, where the patients were recruited from two New Zealand National Diabetic Screening programs; a regional community Optometrist based provider and a Central Auckland DHB provider, are presented. Each of these two programs serve different communities employing a variety of cameras in their screening service. The aim of this study was to establish the efficacy of THEIA™, regardless of the type of fundus camera being used for, or location of the screening centre [5]. In this paper, the results of a bespoke AI are presented, one that was developed to provide primary screening of diabetic retinopathy to augment the existing DR screening program in New Zealand; one that both accurately represents the real-world DR screening environment and is representative of the patients it is designed to serve.
Methods
Study population
This was a prospective study, where participants were recruited from two separate clinics that are participating in the New Zealand Diabetic Screening program. One is a large urban tertiary DHB clinic, the other clinic was located in a provincial optometric practice. The central DHB service used a variety of 45 degree non mydriatic cameras at its different sites; Canon DGi (2 units), Canon CR2 (2 units) and Canon CR2 + AF (2 units), while the optometric led centre was using an iCare EIDON camera. The study protocol was approved by the Health and Disability Ethics committee at New Zealand Health and Disability Ethics Committee (20/STH/178) and Counties Manukau Health (CMH-947). The trial is registered on the ANZCTR, Registration number ACTRN12620000488909 and has been issued with the Universal Trial Number (UTN) U1111-1249-7630.
Consecutive patients attending for a publicly funded retinal screening (within the eye hospital or optometric setting) between January 2021 and April 2021, over the age of 21, were invited to participate. The only exclusion were patients who were unable to give their consent. To ensure that there was a sufficient number of patients with diseased images, the study remained open until the desired number of patients with disease had been recruited.
The process of DR screening in New Zealand has been outlined previously [5], but in brief all participants with Type 2 diabetes mellitus (T2DM) were photographed twice in each eye, i.e. one macula-centred and one disk-centred image, and all patients with Type 1 diabetes mellitus (T1DM) were imaged four times in each eye, with an addition two images taken one below the disc and one above the disc. All patients are initially photographed through undilated pupils, pupil dilation being used if the image that was subsequently acquired was deemed by the photographer to be inadequate. At the conclusion of data collection, the images were de-identified and assigned a unique patient ID by an independent technician.
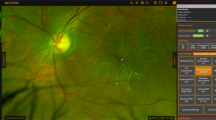
The de-identified images were then passed on to three independent specialists who oversee the DR grading teams at each of the 3 metro Auckland DHBs screening programs. Each graded the entire dataset independently, according to New Zealand Ministry of Health standards [18]. The grading happened simultaneously, and the individual graders were masked to the grades issued by the other two graders. Where there was a discrepancy in the grades issued by the three independent graders a fourth independent, senior retinal specialist was used to adjudicate the outcome. An adjudicated master ‘ground truth’ list was thus created by aggregating the three independent reports. The level of agreement between graders and the adjudicated ground truth was assessed using both a kappa statistic and percentage agreement. This adjudicated data set formed the “ground truth” against which THEIA™ was subsequently independent to the human grading pathway, the de-identified colour images were analysed by the THEIA™ AI platform, by means of uploading images to its dedicated Amazon Web Services (AWS) portal. THEIA™ has been described in detail previously but in brief comprises a Quality assurance AI installed on the capture station and a grading AI that is hosted in the Cloud [5, 6]. The QA function is designed to assess whether the image captured is of acceptable Quality for the suite of grading AI’s to read. If the image is of acceptable quality the user “accepts” the image which is, then sent to the grading AI’s for analysis. If the image is not of adequate quality the user is invited to take further images to secure images that are of sufficient quality. If this is not possible the user is then presented with the choice of abandoning digital imaging and sending the patient for slit lamp review or overriding the inbuilt image QA alert and sending the images for grading regardless.
The THEIA™-generated grades were then compared with the ground truth by way of confusion matrices. Using grades derived from the New Zealand grading system [19], the efficacy of THEIA™ was assessed at the patient-level, using both a simplified binary referrable/non-referable grading scheme and the more global (aggregated) grading scheme of Healthy, mild, more-than-mild (mtmDR) and Sight threatening DR (Table 1). Where a discrepancy existed between the results issued by THEIA™ and the adjudicated ground truth, the images were reassessed by the group who, being masked to the origin of the results and the results issued by THEIA™, were asked to either agree with one of two the outcomes presented. Although the performance of THEIA™ could be reported at either the image, eye or patient level, as it has been designed primarily for use as a Clinician support tool to perform primary grading within a Diabetic eye screening program (DRS) we have chosen to lead with the PATIENT level binary Non referrable/ Referrable data. For sake of transparency all Patient and Eye level data will be presented.
Statistical power calculation
The primary study outcome was the sensitivity and specificity performance of the AI to detect referrable retinopathy. Study success was thus pre-defined as both sensitivity and specificity of the AI system in the New Zealand population. The hypotheses of interest are H0: p < p0 vs: HA: p > p0 where p is the sensitivity or specificity of the AI system and p0 = 75% for the sensitivity endpoint and p0 = 77.5% for the specificity endpoint under the null hypotheses.
The alternative hypotheses were 85% for sensitivity and 82.5% for specificity, reflecting anticipated enrolment numbers and pre-specified service requirements. One-sided testing was further prespecified for both sensitivity and specificity; a one-sided 2.5% Type I error was used resulting in a one-sided 97.5% rejection rule per hypothesis. To preserve Type I error, study success was defined as requiring both null hypotheses to be rejected at the end of the study.
Sample sizes for these hypotheses were calculated for at least 85% power and one-sided 2.5% Type 1 error. This indicated that we required a minimum of 840 participants, at least 149 of whom had referable DR or DMO.
Results
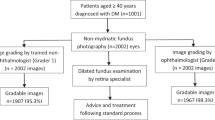
At the time of the study, and to address the large backlog that had resulted from the COVID lockdowns in New Zealand, the diabetic eye screening programs were prioritizing high risk patients with historically suboptimal diabetes control or established retinopathy. It was anticipated that this would result in recruiting a higher number of patients with disease than would usually be the case. Images were read sequentially but because there was both a time lag between the dates the results were issued and the date the patient was recruited and multiple sites were involved, more patients were recruited than the power calculation required (total recruitment 1048). 246 individuals recruited into the study had disease that was deemed to represent referrable disease. Of these 2 had previously treated proliferative DR with extensive pan retinal photocoagulation and in 1 there was an insufficient set of images to be accurately graded. These three participants were therefore excluded from the final analysis. 243 patients with referrable disease were therefore enrolled into this study. The remaining patients (804) had none or minimal disease. As the study had over recruited, to minimize the burden on the grading team, the dataset of patients with none or minimal disease was reduced by random selection to 657 patients to make a total of 900 patients. This curated image dataset was then presented to the studies grading team. The final calculations were therefore based on a total of 900 patients (Fig. 1). THEIA™ managed to grade all the images that were acquired during this study, regardless of the site, camera, or the operator.
Results Binary Non referrable/referrable classification
The Patient-level results, using the simplified 2 class classification Non referrable (R0-R2, M0-M2) vs Referrable (R3-R5, M4-M5), and using the worst R or M outcome in either eye is shown in Table 2. THEIA™ achieved a 100% sensitivity and 98.18% specificity, with the overall accuracy of 98%. Using the binary Non-referrable/referrable classification, the level of agreement between the three individual graders and the gold standard and THEIA™ and the gold standard was extremely high (k values: 0.98, 0.96, and 0.92 respectively for the 3 graders and THEIA k value: 0.95).
The Eye level results for the binary Non referrable/Referrable classification, broken down by the individual R and M grade results, are shown in Supplementary Tables 1–5. The sensitivity and specificity of detecting referrable retinopathy was 98.6% and 92.5% respectively. The sensitivity and specificity of detecting referrable maculopathy was 94.8% and 88.8% respectively.
Results none/mild/more than mild/sight threatening
The Patient-level results; measuring THEIA™ performance against the gold standard with a granular 4 class classification; None (R0, M0), Mild (R1,2; M1,2), more than mild (R3, M3) and Sight threatening (R4,5; M4,5) using the worst R or M outcome in either eye, are illustrated in Table 3. These reveal that THEIA™ tended to marginally over grade disease, but it did not miss any patient with mtmDR disease or worse. THEIA™ issued a lower grade than gold standard in just 1 individual. They were issued with a mtmDR grade when the ground truth was considered to Sight threatening. The level of agreement between the three individual graders and the gold standard using the 4-class classification ranged from k value: 0.96–0.75. The corresponding level of agreement between THEIA™ and the gold standard was k 0.79.
The Eye level results for the 4-class classification, broken down by the individual R and M grade results are shown in Supplementary Tables 1–5. THEIA™ accurately graded the level of retinopathy in 1395/1713 (81.4%) of eyes and accurately graded the level of maculopathy in 1526/1702 (89.7%) of eyes.
THEIA™ demonstrated similar level of proficiency in identifying referable disease, in both the central DHB unit, and the Optometrist led practice (Supplementary Tables 6–8).
Audit of discordant grading
Using the 2 class classification system Referrable vs Non referrable, THEIA™ issued a different grade to the Gold standard adjudicated dataset in just 11 out of 900 patients. In all cases THEIA™ issued a grade that was higher than the gold standard (Table 2, Supplementary Fig. 1). Over grading of maculopathy resulted in 9 of the 11 cases where THEIA™ over graded compared to the gold standard. In 5 cases hard drusen were mistaken for exudate; Pachydrusen (2 cases) and thrombosed microaneurysms (2 cases) were responsible for the remainder. In 2 of the 11 cases where THEIA™ disagreed with the gold standard, THEIA™ issued an R3 grade instead of an R2 grade. In both cases the retinopathy was at the R2/R3 interface. The over-grading can be accounted for by the inbuilt “add up” function within THEIA™ which issued a patient level grade of R3 when the R grade classifier issued an R2 grade in both the disc and macular centred images. THEIA™ missed 4 cases (of 1713 eyes) where the eye level retinopathy was graded at mtmDR and 13 cases (of 1702 eyes) where the eye level maculopathy was graded as mtmDR (Supplementary Table 9).
Other pathologies detected
In addition to screening for DR, our grading team was asked to comment on other sight threatening pathologies. Two patients in the cohort had a hemorrhagic branch retinal occlusion and one patient had a central retinal vein occlusion. Although THEIA™ was not able to identify these diseases specifically, all three were identified by THEIA™ as having “referrable” disease. No other sight threatening pathology was identified in this cohort.
Discussion
While there has been a flurry of research designed to create artificial intelligence tools for screening diabetic retinopathy (DR) or diabetic macular oedema (DMO), few algorithms have been tested prospectively in a real-world clinical environment [20,21,22,23]. This study, was designed to test the efficacy of our previously published algorithm (THEIA™) in a real-world prospective setting of two DR screening programs in New Zealand [5, 6] (Supplementary Table 10); one an urban DHB tertiary hospital screening centre the other a provincial Optometrist led screening centre. In this multi-centre prospective trial of 900 patients, at the patient level when a binary Non referrable/referrable classification was used THEIA™ achieved 100% imageability, 100% sensitivity, 98% specificity, with an overall accuracy of 98% for identifying referable disease when compared to an adjudicated gold standard. When a more granular classification of none. Mild, mtmDR and sight threatening disease was used THEIA™ missed no patient who had referrable disease as defined by either “mtmDR” or Sight threatening disease. The few inconsistent grades between THEIA™ and the adjudicated gold standard were largely a result of drusen; both small and hard drusen, and large pachydrusen [24], being mistaken for exudates. The gold standard adjudicated dataset was derived from grades issued by the senior lead grader in each of the three metro Auckland DHB screening programs. In keeping with their experience, the level of agreement between the individual graders and the adjudicated gold standard (k value: 0.92–0.98) when the data was aggregated into Referrable vs Non referrable disease, was excellent. Although no cases of referrable disease were missed, all three of the human graders marginally under-graded compared to the adjudicated gold standard. This result was not statistically significant. There was a comparable level of agreement between THEIA™ and the adjudicated gold standard (k value: 0.95). In contrast to the human graders, THEIA™ marginally over-graded the images, a result which is in keeping with a tool which is designed with a high sensitivity and thus designed not to miss disease. Overall, these results demonstrate that THEIA™ is both reliable and is as consistent as experienced specialist graders in diagnosing and detecting referrable diabetic retinopathy and maculopathy in the New Zealand (or similar) screening program.
As expected, the accuracy of the level of agreement, for both the human graders and THEIA™ was reduced when the more granular grading system; None Detected, Mild, mtmDR, Sight threatening, was employed. The apparent drop off in performance of both the human graders and THEIA™, (k value: human graders 0.96–0.75; THEIA 0.79), is a function of a number of compounding factors; the imposition of an ordinal scale onto a what is disease continuum leading to an increased probability of a mismatch at what is an artificial boundary of two disease states, and the increased numbers of boundaries that a more granular grading system imposes. To reduce the likelihood of missing disease THEIA™ has therefore been designed with an inbuilt bias to over grade in situations where the disease sits at the boundary threshold of two disease states. Reassuringly THEIA™ accurately predicted the correct grade of retinopathy in 82% cases of retinopathy and 89% cases of maculopathy when these two conditions were considered as different entities. When retinopathy and maculopathy grades were aggregated THEIA™ under graded sight threatening disease in just 7 cases, but in all cases THEIA™ still correctly identified them as “referrable” disease labelling them instead as “mtmDR”.
Compared to other algorithms which have been assessed prospectively in a real world setting [7,8,9, 25], THEIA™ performed very favourably. These results suggest that THEIA™ is capable of providing a very high granularity in the diagnosis of both retinopathy and maculopathy. Furthermore, unlike other clinically tested AIs [26,27,28,29], THEIA™ provides these disease grades based on all images acquired per screening visit with the whole process from image acquisition through to grading being completely automated. While there has been significant interest in developing diabetic retinopathy grading AIs [30], few have been trained to specifically grade diabetic maculopathy as a separate entity [26,27,28,29], this despite diabetic maculopathy being the commonest reason for Ophthalmology referral [31]. The performance of the retinopathy classifier was better than the maculopathy classifier, with most false positives being a result of over grading maculopathy. Grading maculopathy is more challenging than grading retinopathy [32]; firstly, exudate is used as a surrogate marker for oedema, and secondly there are several mimics of exudate, such as drusen, pachydrusen, focal ERM, that are difficult to discern without OCT. To address this issue screening programs in the UK and New Zealand have now started to incorporate OCT into their screening pathways. However, as most DR screening programs still operate an asynchronous model of care, and small hard drusen and focal reflective ERM are easily over looked at the time the patient attends for screening, these pathologies are often not identified until the retinal images are reviewed after the screening event. One advantage of using an AI such as THEIA™, which is capable of grading in real time, is that it facilitates the transition to synchronous models of care where patients can be issued their results at the point of care. An additional benefit of this model is that those patients who the AI identifies as having “suspected” maculopathy can be immediately imaged with OCT. This image could be read on the spot if telehealth support is available, or later if not. In either case there is no requirement for the patient to return as all the data required to grade their disease has been acquired.
THEIA™ has been designed primarily as a clinician assist primary triage tool. As such, it has been designed with an ultra-high sensitivity to ensure that sight threatening disease is not missed. In its previous configuration, THEIA™ achieved this at the expense of a modest specificity [5]. With a modification to the algorithm, the current version of THEIA™ preserved its ultra-high sensitivity while achieving a specificity higher than 95%. Whilst there was still a tendency for THEIA™ to over grade the issue of false positives is not overly troublesome because being a primary grading support tool it simply means that borderline images need to be read by a member of the grading team. The trade-off for the tendency to over grade is an ultra-high sensitivity and negative predictive value. Consequently, if THEIA™ grades an image as having no significant disease those responsible for the diabetic eye screening program can be confident that no significant disease has been missed. As most patients undergoing screening have minimal or no disease, we believe that the trade-off between “no disease missed” and a small number of false positives is reasonable. In this trial, four different camera types were used in multiple clinical settings; these included an iCare Eidon camera (confocal scanning laser ophthalmoscopy technology), and a variety of Canon cameras (conventional flash photography technology). THEIA™’s performance was unaffected by the camera type used or the shape and size of the image (Supplementary Tables 8 & 9). It also coped well with a number of artifacts on the real-world images including a central bright halo that was generated by one camera, and a random assortment of dot artefacts that appeared in a consistent location from another camera (Supplementary Fig. 2).
Whilst an accurate Algorithm is clearly important, there are several diverse issues that need to be addressed before AI can be safely incorporated into diabetic eye screening programs. These include but are not limited to equity, consent data privacy and stakeholder acceptance [17]. We have recently explored the attitude of patients undergoing retinal screening to the concept of using AI to read the retinal images acquired at the time of screening [33]. We found that although there is low awareness of clinical AI applications among our participants, most (78%) were receptive towards the implementation of AI in diabetic eye screening. In line with other similar surveys [34] there was a strong preference towards continual involvement of clinicians in the screening process and it is likely there will need to remain an option for those who prefer the service to be delivered manually [33]. These findings suggest that if clinical algorithm’s like THEIA™ are to be acceptable to stakeholders they will need to be deployed as primary grading support tools that augment the clinical teams at the point of care. Although a separate cost analysis of implementing THEIA™ was not part of this project, a team from Singapore have estimated that the adoption of a primary grading AI system, similar to THEIA™, would reduce the costs of delivering their existing DRS program by 20% [35].
The principal limitation of THEIA™ is that it cannot reliably identify other eye diseases that can present at the time of diabetic screening, such as glaucomatous optic neuropathy and age-related macular degeneration. Three patients in the current study had a significant retinal vein occlusion that was flagged up as significant retinopathy. It would also be reasonable to expect that haemorrhagic neovascular macular degeneration to be similarly identified. The Auckland DR screening program systematically records all other pathologies that are detected during routine screening. A recent analysis of this data revealed that only severe hypertensive retinopathy, retinal vein occlusion and macular degeneration are sufficiently important to justify systematic detection during routine diabetic eye screening [36]. Severe hypertensive retinopathy and many cases of advanced macular degeneration would already be picked up and flagged up by THEIA™ as mtmDR or Sight Threatening disease. Incorporating an AI classifier capable of detecting glaucoma suspects and intermediate and late AMD in addition to DR, would add further capability to THEIA™. Another potential limitation of AI is the ability of the algorithm to generalize to the population in which it is intended to be used. The population demographic that THEIA™ was trained has been described elsewhere [5]. Although the MoH in New Zealand does not keep a register of people living with diabetes, the Virtual Diabetes Register (VDR) [37] gives an estimate of the prevalence of diabetes in NZ, broken down by region and ethnicity. Comparison of the relative proportions of people living with diabetes in both the cohort who comprised the previously published retrospective study [5] and the current prospective study are similar to those reported in the VDR (Māori 18%, Pacific peoples 16%, Indian 6%, European/Asian/others 57%). We are therefore confident that our data is representative of the wider population of people living with diabetes in New Zealand and that the result of the current study therefore indicates that THEIA™ has successfully generalized to the population in New Zealand living with diabetes.
In conclusion, this multi-centre prospective trial demonstrates that THEIA™ is capable of detecting DR and DMO with a very high degree of accuracy, while providing a high level of granularity in grading. As such, and with appropriate oversight and audit, these results indicate that THEIA™ could be safely deployed within established diabetic screening programs to augment the expertise of the clinicians, increasing overall screening capacity while reducing costs per unit screen.
Novelty statement
THEIA is proven to be the most accurate algorithm of its kind, through a double-blind prospective multi-centre trial. THIEA provides the highest level of disease diagnosis granularity, which is essential for early detection and timely intervention. THEIA provides an automated decision rule to ensure rapid, accurate classification of the large proportion of normal images from the few with abnormal features for prompt, accurate clinical grading, but not to replicate a screening program.
Summary
What was known before
-
While there has been a flurry of research designed to create artificial intelligence tools for screening diabetic retinopathy (DR) or diabetic macular oedema (DMO), few algorithms have been tested prospectively in a real-world clinical environment. This study, was designed to test the efficacy of our previously published algorithm (THEIA™) in a real-world prospective setting of two DR screening programs in New Zealand.
What this study adds
-
This multi-centre prospective trial showed that THEIA™ did not miss referable disease when screening for diabetic retinopathy and maculopathy. It also had a very high level of granularity in reporting the disease level. As THEIA™ has been tested on a variety of cameras, operating in a range of clinics (rural/urban, ophthalmologist-led/optometrist-led), we believe that it will be a suitable addition to a public diabetic screening program.
Data availability
The data was collected prospectively from consented participants [ANZCTR - ACTRN12620000488909 - Universal Trial Number (UTN) U1111-1249-7630.], specifically for this study and can not be released to external bodies without their consent.
References
Bellemo V, Lim G, Rim TH, Tan GS, Cheung CY, Sadda S, et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diabetes Rep. 2019;19:72.
Lee A, Taylor P, Kalpathy-Cramer J, Tufail A. Machine learning has arrived! Ophthalmology. 2017;124:1726–8.
Nørgaard MF, Grauslund J. Automated screening for diabetic retinopathy–a systematic review. Ophthalmic Res. 2018;60:9–17.
Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77.
Vaghefi E, Yang S, Xie L, Hill S, Schmiedel O, Murphy R, et al. THEIA™ development, and testing of artificial intelligence‐based primary triage of diabetic retinopathy screening images in New Zealand. Diabet Med. 2021;38:e14386.
Xie L, Yang S, Squirrell D, Vaghefi E. Towards implementation of AI in New Zealand national diabetic screening program: Cloud-based, robust, and bespoke. Plos One. 2020;15:e0225015.
Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Med. 2018;1:1–8.
Ting DSW, Cheung CY-L, Lim G, Tan GSW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–23.
Bhaskaranand M, Ramachandra C, Bhat S, Cuadros J, Nittala MG, Sadda SR, et al. The value of automated diabetic retinopathy screening with the EyeArt system: a study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol Therapeutics. 2019;21:635–43.
Scanlon PH. Screening intervals for diabetic retinopathy and implications for care. Curr diabetes Rep. 2017;17:96.
Nguyen HV, Tan GSW, Tapp RJ, Mital S, Ting DSW, Wong HT, et al. Cost-effectiveness of a national telemedicine diabetic retinopathy screening program in Singapore. Ophthalmology. 2016;123:2571–80.
Came H, O’Sullivan D, Kidd J, McCreanor T. The Waitangi Tribunal’s WAI 2575 report: implications for decolonizing health systems. Health Hum Rights. 2020;22:209.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Xie Y, Nguyen Q, Bellemo V, Yip MY, Lee XQ, Hamzah H, et al. Cost-effectiveness analysis of an artificial intelligence-assisted deep learning system implemented in the national tele-medicine diabetic retinopathy screening in Singapore. Investigative Ophthalmol Vis Sci. 2019;60:5471–71.
Xie Y, Gunasekeran DV, Balaskas K, Keane PA, Sim DA, Bachmann LM, et al. Health economic and safety considerations for artificial intelligence applications in diabetic retinopathy screening. Transl Vis Sci Technol. 2020;9:22–2.
Lee AY, Yanagihara RT, Lee CS, Blazes M, Jung HC, Chee YE, et al. Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care. 2021;44:1168–75.
Chu A, Squirrell D, Phillips AM, Vaghefi E. Essentials of a robust deep learning system for diabetic retinopathy screening: a systematic literature review. J Ophthalmol. 2020;2020.
Zealand MoH-N. Diabetic Retinal Screening, Grading, Monitoring and Referral Guidance. In: Diabetes, ed.^Vol 1. health.govt.nz: Ministry of Health; 2016.
Health Mo. Diabetic Retinal Screening, Grading, Monitoring and Referral Guidance. 2016.
Heydon P, Egan C, Bolter L, Chambers R, Anderson J, Aldington S, et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30,000 patients. Br J Ophthalmol. 2021;105:723–8.
Zhang Y, Shi J, Peng Y, Zhao Z, Zheng Q, Wang Z, et al. Artificial intelligence-enabled screening for diabetic retinopathy: a real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. 2020;8:e001596.
Shah A, Clarida W, Amelon R, Hernaez-Ortega MC, Navea A, Morales-Olivas J, et al. Validation of automated screening for referable diabetic retinopathy with an autonomous diagnostic artificial intelligence system in a Spanish population. J Diabetes Sci Technol. 2020:1932296820906212.
Bellemo V, Lim ZW, Lim G, Nguyen QD, Xie Y, Yip MY, et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. Lancet Digital Health. 2019;1:e35–44.
Zhang X, Sivaprasad S. Drusen and pachydrusen: the definition, pathogenesis, and clinical significance. Eye. 2021;35:121–33.
Ipp E, Liljenquist D, Bode B, Shah VN, Silverstein S, Regillo CD, et al. Pivotal Evaluation of an Artificial Intelligence System for Autonomous Detection of Referrable and Vision-Threatening Diabetic Retinopathy. JAMA Netw Open. 2021;4:e2134254–254.
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investigative Ophthalmol Vis Sci. 2016;57:5200–6.
Gulshan V, Rajan RP, Widner K, Wu D, Wubbels P, Rhodes T, et al. Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019;137:987–93.
Krause J, Gulshan V, Rahimy E, Karth P, Widner K, Corrado GS, et al. Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy. Ophthalmology. 2018;125:1264–72.
Ramachandran N, Hong SC, Sime MJ, Wilson GA. Diabetic retinopathy screening using deep neural network. Clin Exp Ophthalmol. 2018;46:412–6.
Ou WCWC The Promise of Deep Learning in Retina. Retina Specialist. Available at: https://www.retina-specialist.com/article/the-promise-of-deep-learning--in-retina-1-1, 2022.
Ruta L, Magliano D, Lemesurier R, Taylor H, Zimmet P, Shaw J. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med. 2013;30:387–98.
Scanlon PH. The English national screening programme for diabetic retinopathy 2003–2016. Acta Diabetologica. 2017;54:515–25.
Yap A, Wilkinson B, Chen E, Han L, Vaghefi E, Galloway C, et al. Patients’ perceptions of artificial intelligence in diabetic eye screening. 2022 RANZCO. Brisbane - Australia: The Royal Australian and New Zealand College of Ophthalmologists; 2022.
Scheetz J, Koca D, McGuinness M, Holloway E, Tan Z, Zhu Z, et al. Real-world artificial intelligence-based opportunistic screening for diabetic retinopathy in endocrinology and indigenous healthcare settings in Australia. Sci Rep. 2021;11:1–11.
Xie Y, Nguyen QD, Hamzah H, Lim G, Bellemo V, Gunasekeran DV, et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digital Health. 2020;2:e240–9.
Ramachandran N, Schmiedel O, Vaghefi E, Hill S, Wilson G, Squirrell D. Evaluation of the prevalence of non-diabetic eye disease detected at first screen from a single region diabetic retinopathy screening program; a cross-sectional cohort study in Auckland, New Zealand. BMJ Open. 2021; In-Press.
Ministry of Health NZ. Virtual Diabetes Register (VDR). Ministry of Health, New Zealand. Available at: https://www.health.govt.nz/our-work/diseases-and-conditions/diabetes/about-diabetes/virtual-diabetes-register-vdr?msclkid=de768615ce7d11ec8ae563ee11a7fff8, 2022.
Acknowledgements
We wish to acknowledge the Diabetes eye screening program of Counties Manukau District Health Board and Naylor Palmer optometrists for hosting the trial.
Funding
This work was funded by Ministry of Business, Innovation and Education of New Zealand (UOAX1805 - 3715780). Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
EV proposed the original research, developed the original methodology and developed the first draft of the manuscript. SY, DH & LX (equal contribution) supervised the technical aspect of the trial. AY assisted in the clinical data collection and writing of the manuscript. DS supervised the clinical aspects of the trial and reviewed the manuscript. OS supported clinical aspects of the study and reviewed the manuscript. JM reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
EV and DS are co-founders of Toku Eyes®, which is a start-up out of The University of Auckland, looking into commercialization of this artificial intelligence system (THEIA™) in New Zealand. No other conflict of interest for co-authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaghefi, E., Yang, S., Xie, L. et al. A multi-centre prospective evaluation of THEIA™ to detect diabetic retinopathy (DR) and diabetic macular oedema (DMO) in the New Zealand screening program. Eye 37, 1683–1689 (2023). https://doi.org/10.1038/s41433-022-02217-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02217-w