Abstract
Objective
To explore the presence of residual emulsified silicone oil (SO) droplets in patients with rhegmatogenous retinal detachment (RRD) and their possible risk factors.
Methods
Patients who underwent primary pars plana vitrectomy with SO injection for RRD and SO removal at the same eye centre were included. Approximately 10 weeks after SO removal, B-scan ultrasonography was performed, and using ImageJ, the silicone oil index (SOI) was measured, and its possible correlations with other clinical factors were explored.
Results
A total of 101 eyes were included. Residual SO particles were found in all the patients (100%), and the mean SOI was 4.04% ± 5.16% (range 0.06%–19.88%). Multiple linear regression revealed that, among all the clinical factors, axial length (AL) and ocular hypertension (intraocular pressure (IOP) > 21 mmHg or the use of antiglaucoma medications) before SO removal were positively and significantly associated with the SOI (all P < 0.05). Patients with ocular hypertension after SO removal had a higher SOI, a longer SO duration, a higher IOP before SO removal and a longer AL than those without (all P < 0.05).
Conclusions
Patients with a larger AL and higher IOP before SO removal were more prone to have more residual SO droplets, which might in turn lead to an elevated IOP. In these eyes, thorough irrigation or repeated fluid-air exchange might be necessary.
Similar content being viewed by others
Introduction
Silicone oil (SO) was first introduced by Cibis et al. [1] in the 1960s and is now widely used in the management of complicated retinal detachment. However, complications such as SO emulsification [2] and glaucoma [3,4,5,6,7,8] have long been a reported concern. Small residual emulsified SO droplets in the vitreous cavity after SO removal have also been widely reported, especially during ultrasound B-scan exams [9, 10]. Recently, using B-scan images and ImageJ software, Stalmans et al. [11] and Shiihara et al. [12] introduced a method for evaluating residual emulsified SO droplets in the vitreous cavity. These studies encouraged us to explore the presence of residual SO in these eyes, but the number of cases in the primary reports is limited [12]. In this study, a relatively large number of patients were included, and using the methods, the presence of residual emulsified SO droplets in the vitreous cavity was studied, and its possible correlation with other clinical factors was explored. This might help to improve our knowledge of residual SO in these eyes and to identify patients who might need special attention.
Materials and methods
Study subjects and ethics statement
This was a single-centre, observational, cross-sectional study. Patients who underwent primary pars plana vitrectomy (PPV) with SO injection for rhegmatogenous retinal detachment (RRD), followed by SO removal at the Eye and ENT Hospital of Fudan University between January 2019 and January 2022, were enrolled in the study.
The study was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University and conformed to the tenets of the Declaration of Helsinki. All the patients signed informed consent forms.
Patients who underwent a minimum of 8 weeks of follow-up after SO removal and whose retinas were still attached were included. Patients with a history of diabetes mellitus, previous SO injection, previous intraocular disease (except cataract) before RRD (e.g., glaucoma, uveitis), or elevated intraocular pressure (IOP > 21 mmHg), and who were age < 18 years at the time of primary PPV for RRD were excluded from the study.
Main ophthalmic measurements
At approximately 10(8–12) weeks after SO removal, each patient underwent a thorough ophthalmic examination, which included assessment of the best-corrected visual acuity (BCVA; logarithm of the minimal angle of resolution[logMAR]), slit-lamp microscopy, dilated fundus examination with a noncontact lens (Maxfield 84 Diopter; Ocular, USA), measurement of IOP by noncontact tonometry, measurement of axial length (AL) using IOL master (version 3.01; Carl Zeiss Meditec, Jena, Germany), and a B-scan exam (detailed below). Their demographic features and clinical histories were also collected, including age, sex, number of PPVs, history of ocular trauma, combined procedures during SO removal, duration of SO in situ, lens status, with/without ocular hypertension before and after SO removal (at the time of B-scan) and others. Ocular hypertension was defined as those with IOP higher than 21 mmHg, or the ones using antiglaucoma medications.
SO removal surgical procedures
SO (5700 cSt; Bausch & Lomb Inc., Rochester, NY, USA) was used in all cases. During SO removal, after the main bulk of SO was removed by active suction, passive drainage was performed, during which a 23-gauge back flute needle was inserted into the vitreous cavity, and the fluid was drained for 15 min while the infusion pressure was set to 20–25 mmHg (Constellation 5000 Vision System, Alcon Laboratories, Inc., Fort Worth, TX, USA).
B-scan examination and analysis
B-scan was performed using a 10-MHz B-Scan Probe (AVISO, Quantel Medical, France) with the following settings: Gain = 105 dB, Dyn = 55 dB, Tgc = 10 dB. The patients were asked to look in the nasal direction, and the probe was oriented vertically to the temporal sclera. A clear image containing the largest vitreous cavity and lacking ultrasound reverberations due to poor contact between the probe and the eyeball was taken and saved for further analysis.
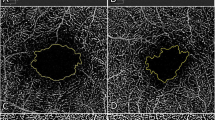
Evaluation of the residual silicone oil in situ was performed according to the method described by Stalmans et al. [11] and Shiihara et al. [12]. Briefly, the images were first converted to an 8-bit format. A threshold was set to then convert the image to black and white with a dark background. Then, a polygon was drawn over the vitreous cavity, within which the area of all particles measuring between 25 and 1000 pixels was automatically summed (Fig. 1). The SO index (SOI) was calculated by the following formula:
For the first 20 images, intraobserver repeatability and interobserver reproducibility were evaluated by two observers, who each measured the same scan twice. The intraclass correlation (ICC) coefficient was used to assess repeatability and reproducibility.
Data and statistical analysis
All analyses were performed using SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous data are expressed as the mean ± standard deviation. ICC coefficients were used to assess repeatability and reproducibility; an ICC coefficient of 0.81–1.00 indicates almost perfect agreement between repeated measurements, and values <0.40 indicate poor to fair agreement.
Spearman’s correlation coefficient, the Mann–Whitney U test and multiple linear regression were used to assess correlations between clinical characteristics and the SOI. The Mann–Whitney U test was also used to assess the differences between patients with/without ocular hypertension after SO removal (at the time of B-scan examination). Statistical significance was defined as a P value <0.05.
Results
A total of 101 eyes (64 right eyes) in 101 patients (62 males) were included in this study. The mean age was 51.29 ± 14.96 years (range 18–86 years), the mean duration of SO in situ was 23.79 ± 12.30 weeks (range 7.00–104.00 weeks), and the mean AL was 26.43 ± 2.76 mm (range 22.02–33.62 mm). At the time of PPV and SO injection, nine patients had choroidal detachment, 15 had a history of ocular trauma, and five had giant retinal tears. For the SO removal procedure, in 69 patients, the procedure was combined with other operations (e.g., phacoemulsification, intraocular lens implantation, or epiretinal membrane peeling). The status of the lens after SO removal was aphakic in 35 eyes, phakic in 29 and pseudophakic in 37.
Residual SO was detected by B-scan in all 101 eyes (100%). The average SOI was 4.04% ± 5.16% (range 0.06–19.88%). The mean ICC coefficients for intraobserver repeatability and interobserver reproducibility were as high as 0.974 and 0.980 (both P < 0.001), respectively, which were considered excellent.
Univariate analysis showed that the duration of SO in situ, the AL, the presence of ocular hypertension before SO removal, and the presence of ocular hypertension after SO removal were significantly correlated with the SOI (all P < 0.05), while all other parameters were not (all P > 0.05) (Table 1). Multiple linear regression revealed that the AL and ocular hypertension before SO removal were positively associated with the SOI (both P < 0.05) (Table 2).
After SO removal (at the time of the B-scan), 32 patients had ocular hypertension. These patients had a higher SOI, a larger AL, a higher IOP before SO removal and a longer SO duration than patients without ocular hypertension (all P < 0.05), and all other parameters were similar between the two groups (all P > 0.05) (Table 3 and Fig. 2).
Discussion
In this study, using B-scan ultrasonography and ImageJ, residual emulsified SO droplets in the vitreous cavity were studied. A rather large group of patients with RRD was included, one type of SO was used, and B-scan examinations were performed by one ophthalmologist, so the results in this study were more reliable. Residual SO droplets were found in all the patients, and the SOI (the proportional area of SO droplets on B-scan imaging) was positively correlated with AL and ocular hypertension.
Residual emulsified SO droplets in the anterior chamber or the vitreous cavity after SO removal have been observed during slit lamp examinations for many years [13, 10]. Henneken and Machemer [9] first reported highly reflective objects during B-scans. Using ImageJ, Shiihara et al. [12] and Stalmans et al. [11] described a method for quantifying these objects. Compared to other methods, such as the Coulter counter, this method is noninvasive, and could perform a highly cost-effective and highly reproducible in vivo measurement. Above all, this method does not require any specific hardware or software. This provides us with a new way to evaluate residual SO droplets in the clinical setting. However, previous studies only included a limited number of patients with a variety of retinal diseases; as a result, the relationships between residual SO and clinical factors was not fully explored. Therefore, in this study, a relatively large group of patients with RRD were included, and to rule out examiner influence, a B-scan exam was performed by a fixed examiner.
A B-scan exam found emulsified SO droplets in all eyes. This is in accordance with clinical experience and in previous studies [12, 14]. The SOI at this time was 4.04%, which is within the range of previous reports (3.2–7.4%) [12, 14]. This, in another way, suggested the reliability of this method.
In this study, AL, ocular hypertension and SO duration were significantly and positively correlated with the SOI. Recently, Shiihara et al. also reported a correlation between high SOI and AL [12]. The reason for this is not fully understood, but it is possible that eyes with a larger AL have a greater vitreous cavity and intraocular surface area, so a greater volume of SO was injected in these eyes, and the interface between the SO and intraocular fluid was larger. As a result, the surfactants (surface active agents) had a greater opportunity to interact with SO, thus increasing the risk of emulsification. Additionally, in our previous study, we found that the AL was positively and significantly correlated with the total grade of SO emulsification before SO removal [15]. Therefore, more emulsified SO droplets in eyes with greater ALs might be the reason behind this finding. Another factor influencing emulsification is duration [16]. In Federman et al.’s [2] study, the percentages of SO emulsification at 1 month, 2 months, 3 months, 6 months and 1 year were 1%, 6%, 11%, 85% and 100%, respectively. With the same SO removal efficiency, more emulsification means more residual SO, which could explain the positive correlation between the AL, duration of SO tamponade and the SOI found in this study.
The correlation between SO emulsification and elevated IOP has been widely noticed, and many reasons have been proposed. One of them is the migration of SO droplets from the vitreous cavity, which could directly obstruct the trabecular meshwork or cause inflammation [16]. Long-term contact between the emulsified SO droplets and the trabecular meshwork might also result in sclerosis and collapse of the trabecular meshwork. The correlation between SOI and ocular hypertension was also found here. Additionally, eyes with ocular hypertension after SO had a higher SOI than eyes without. These findings, once again, strengthened the important relationship between emulsified SO droplets and a high IOP.
According to the finding this time, special attention must be given to patients with high-risk factors (such as longer AL, ocular hypertension before SO removal), and thorough irrigation or repeated fluid-air exchange should be considered to avoid residual SO droplets, which might in turn lead to high IOP again after SO removal.
Our study was limited by its cross-sectional design, and only one type of SO – 5700 cSt was studied. Additionally, previous studies have revealed that most emulsified droplets are too small in size to be seen on clinical examination [17,18,19], and resolution of B-scan(especially the 10 MHz used here) is limited, so small droplets could be missed. And, B-scan can only detect SO droplets in the vitreous cavity and cannot detect those that have adhered to the retina or anterior segment. Furthermore, the results of B-scan might be operator dependent, and the SOI only provided the sum-up area of all droplets, the size distribution was not available like other method, for example Coulter counter.
In conclusion, B-scan imaging and ImageJ were used to study residual SO droplets in the vitreous cavity in RRD patients treated with SO tamponade. More droplets were found in patients with greater ALs and those with ocular hypertension before SO removal, and special attention should be given to these individuals during SO removal to ensure more complete removal. Future long term follow-up study should be carried out to see if the SOI changes with time, and to compare the findings of different method - B-scan, Coulter Counter and UBM in the evaluation of SO emulsification. These studies might further improve our knowledge in this field.
Summary
What was known before
-
Recently, using B-scan images and ImageJ software, researchers introduced a method for evaluating residual emulsified SO droplets in the vitreous cavity.
What this study adds
-
Patients with a larger AL and higher IOP before SO removal were more prone to have more residual SO droplets, which might in turn lead to an elevated IOP.
Data availability
The research data used to support the findings of this study are included within the article.
References
Cibis PA, Becker B, Okun E, Canaan S. The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol. 1962;68:590–9.
Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology 1988;95:870–6.
McCuen BW 2nd, de Juan E Jr, Landers MB 3rd, Machemer R. Silicone oil in vitreoretinal surgery. Part 2: Results and complications. Retina 1985;5:198–205.
Casswell AG, Gregor ZJ. Silicone oil removal. I. The effect on the complications of silicone oil. Br J Ophthalmol. 1987;71:893–7.
Riedel KG, Gabel VP, Neubauer L, Kampik A, Lund OE. Intravitreal silicone oil injection: complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol. 1990;228:19–23.
Honavar SG, Goyal M, Majji AB, Sen PK, Naduvilath T, Dandona L. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology 1999;106:169–76. discussion 77
Al-Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil injection. J Glaucoma. 2005;14:40–6.
Ichhpujani P, Jindal A, Jay Katz L. Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol. 2009;247:1585–93.
Henneken A, Machemer R. Ultrasonographic ‘oil droplet phenomenon’. Arch Ophthalmol. 1991;109:320.
Spaide RF, Chung JE, Fisher YL. Ultrasound detection of silicone oil after its removal in retinal reattachment surgery. Retina 2005;25:943–5.
Stalmans P, Pinxten A-M, Wong DS. Cohort Safety and Efficacy Study of Siluron2000 Emulsification-Resistant Silicone Oil and F4h5 in the Treatment of Full-Thickness Macular Hole. Retina 2015;35:2558–66.
Shiihara H, Terasaki H, Yoshihara N, Shirasawa M, Otsuka H, Yamashita T, et al. Amount of residual silicone oil in vitreous cavity is significantly correlated with axial length. Retina 2016;36:181–7.
Issa R, Xia T, Zarbin MA, Bhagat N. Silicone oil removal: post-operative complications. Eye (Lond). 2020;34:537–43.
Shiihara H, Sakamoto T, Terasaki H, Yamashita T, Yoshihara N, Okamoto F, et al. Effect of fluid-air exchange on reducing residual silicone oil after silicone oil removal. Graefes Arch Clin Exp Ophthalmol. 2017;255:1697–704.
Zhao H, Yu J, Zong Y, Jiang C, Zhu H, Xu G. Characteristics of silicone oil emulsification after vitrectomy for rhegmatogenous retinal detachment: an ultrasound biomicroscopy study. Front Med. 2022;8:794786.
Miller JB, Papakostas TD, Vavvas DG. Complications of emulsified silicone oil after retinal detachment repair. Semin Ophthalmol. 2014;29:312–8.
Chan YK, Cheung N, Chan WS, Wong D. Quantifying silicone oil emulsification in patients: are we only seeing the tip of the iceberg? Graefes Arch Clin Exp Ophthalmol. 2015;253:1671–5.
Yu J, Zong Y, Jiang C, Zhu H, Deng G, Xu G. Silicone oil emulsification after vitrectomy for rhegmatogenous retinal detachment. J Ophthalmol. 2020;2020:6940625.
Yu J, Zong Y, Tan Y, Jiang C, Xu G. Comparison of repeated fluid-air exchange and passive drainage for removing residual emulsified silicone oil droplets. J Ophthalmol. 2020;2020:8184607.
Funding
Publication of this article was supported, in part, by research grants from the National Key Research & Development Plan (2017YFC0108200), the Shanghai Committee of Science and Technology (19441900900, 201409006800), National Natural Science Foundation of China (82070980).
Author information
Authors and Affiliations
Contributions
HZ, TC and KW analyzed the patient data and made major contributions for writing the manuscript. JY and YZ performed the literature review for similar topics and made major contributions to acquisition and interpretation of data. CJ, HZ, QC, and GX made substantial contributions to conception and design this study. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, H., Cheng, T., Wu, K. et al. Silicone oil residual after vitrectomy for rhegmatogenous retinal detachment. Eye 37, 1829–1833 (2023). https://doi.org/10.1038/s41433-022-02210-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02210-3