Abstract
Introduction
Proton beam therapy has been utilised for the treatment of uveal melanoma in the UK for over 30 years, undertaken under a single centre. In the UK, all ocular tumours are treated at one of four centres. We aimed to understand the variation in referral patterns to the UK proton service, capturing all uveal melanoma patients treated with this modality.
Methods
Retrospective analysis of data regarding all patients treated at the Clatterbridge Proton service between January 2004 and December 2014.
Results
A total of 1084 patients with uveal melanoma were treated. The mean age was 57 years (range 9–90 years), basal diameter of 11.5 mm (range 2.0–23.4 mm) and tumour thickness of 3.9 mm (range 0.1–15.4 mm). The majority were TNM stage I (39%) or II (36%). The distance to the optic nerve varied from 0 to 24.5 mm with 148 (14%) of patients having ciliary body involvement. There were variations in the phenotypic characteristic of the tumours treated with protons from different centres, with London referring predominantly small tumours at the posterior pole, Glasgow referring large tumours often at the ciliary body and Liverpool sending a mix of these groups.
Discussion
In the UK, common indications for the use of proton treatment in uveal melanoma include small tumours in the posterior pole poorly accessible for plaque treatment (adjacent to the disc), tumours at the posterior pole affecting the fovea and large anterior tumours traditionally too large for brachytherapy. This is the first UK-wide audit enabling the capture of all patients treated at the single proton centre.
Similar content being viewed by others
Introduction
Proton beam radiotherapy (PBR) is a highly conformal radiotherapy offering considerable advantages over conventional teletherapy with x-rays. In particular, the radiation dose received by adjacent critical or healthy tissue is significantly lower compared to photons [1]. Significant disadvantages are the much higher capital and running costs and the necessity for technically advanced facilities designed to optimise patient treatment. In addition, there is a paucity of clinical outcome data supporting the use of protons over other types of radiotherapy for a number of potential treatment indications [2]. Despite the theoretical advantages of proton beam treatments and its introduction into clinical practice in the 1950s, it is currently used in less than 1% of all tumours undergoing radiotherapy across all disciplines of oncology [1, 3]. However, the percentage is increasing; in the UK, a recent major investment by the government through the Department of Health has led to the construction of two new PBR treatment centres, focussing mainly on head/neck and central nervous system tumours in paediatric patients, teenagers and young adults [4].
In contrast to other oncology subspecialities, PBR has established itself as one of the core treatments of ocular tumours worldwide. Since the first treatment in the USA in 1975, a number of centres specifically designed for eye tumours have been established; based on previously published series, it can be projected that now more than 43,000 patients have been treated with this method worldwide [5, 6]. Multiple clinical studies have been published, demonstrating excellent results regarding the major parameters in judging successful treatment of uveal melanoma (UM): eye preservation, tumour recurrence and vision [7, 8]. Some recent studies suggested that early treatment of UM may have a beneficial effect on survival [9,10,11]. PBR could be of particular advantage, as the damaging side effects on vision caused by the destruction of non-malignant tissue surrounding the tumour seem to be less pronounced in small T1 tumours compared to other methods of radiotherapy [12]. This could lower the threshold for early treatment of small tumours near the fovea and disc.
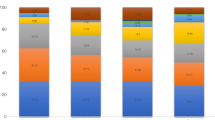
In the UK, between 650 and 700 new cases of UM are treated per year (Fig. 1). Four centres within the National Health Service (NHS), in Glasgow, Liverpool, London and Sheffield, have been commissioned as Highly Specialized Services for Adult Ocular Oncology. In the UK, about 70% of patients undergo radiotherapy as the initial treatment method, with the remaining 30% treated surgically with enucleation or surgical resection (Fig. 1). Three types of radiotherapy are currently used in the UK to treat UM: Ruthenium-106 plaque brachytherapy is the most commonly applied method in 40–50% of all new patients. PBR is used to treat between 20 and 40%. Stereotactic radiotherapy is performed in the Sheffield Centre and is used in approximately 4% of all new patients [13,14,15].
In the UK, the cyclotron service at Clatterbridge was established as the first hospital-based cyclotron unit for patient treatment in 1989 [14]. The use of PBR varies from centre to centre and from country to country, mainly depending on the availability of proton beam facilities and the experience with the method.
This study was conducted as a national audit of referral patterns across the four centres in the UK, for proton treatment over a 10-year period.
Materials and methods
Patients
Inclusion criteria: in this retrospective study, clinical notes from all patients treated at the Clatterbridge proton beam service from January 2004 to December 2014 were reviewed. All patients with choroidal or ciliary body melanoma treated by PBR as their primary treatment were included.
Exclusion criteria: iris and ocular surface melanomas were excluded as the AJCC tumour classification and potential for metastatic disease are significantly different from ciliary body and choroidal lesions. Non-UK resident patients were also excluded as their follow-up would be limited.
All baseline visits consisted of a complete clinical ophthalmological examination, colour tumour photography and ultrasonography. Local patient records as well as Clatterbridge recorded data were utilised in unison and collected by each oncology centre, and subsequently collated. Parameters recorded include age, gender, best-corrected visual acuity, tumour dimensions (largest tumour diameter, height), location of anterior and posterior tumour margin (iris, ciliary body, pre-post equatorial choroid), ciliary body involvement, distance to fovea, disc involvement, extraocular spread, infiltration, retinal detachment, TNM staging and tumour volume.
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Institutional Review Board/Ethics committee approval was not required due to the retrospective nature of this audit. Statistical analysis was undertaken with a single-factor ANOVA to assess the significance of differences in tumour characteristics between centres. P values <0.05 were considered significant.
Results
Over the study period, 1084 patients were treated for choroidal or ciliary body melanoma in the UK with PBR as primary treatment at the Clatterbridge Centre for Oncology (now The Clatterbridge Cancer Centre). Patients had a mean age of 57 years with a male:female ratio of 1.15:1 (Table 1). The right eye was affected in 563 patients (51.9%). The baseline visual acuity of the affected eye was ≧logMar 0.0 in 463 (42.7%), 0.1–0.3 in 301 (27.8%), 0.4–0.9 in 219 (20.2%) and ≦1.0 in 101 (9.3%).
The anterior tumour margin was located post equatorial in 503 (46.4%), between the equator and pars plicata in 358 (33.0%) and involved the angle/iris in 223 (20.6%). The posterior tumour margin was located at the disc in 384 (35.4%), at the fovea in 362 (33.4%), at 1–2-disc diameters away from the disc or fovea in 224 (20.7%), and more anteriorly in 114 (10.5%).
Tumours treated included very small tumours (LBD smallest 2.0 mm and thickness 0.1 mm) and very large tumours (largest LBD 23.4 mm and thickness 15.4 mm). Nearly 75% (n = 811) were TI–IIa tumour category.
As per the COMS definitions, 423 patients (39%) had small tumours (LBD ≤16 mm and thickness ≤2.5 mm), 455 (42%) had medium tumours (LBD ≤16 mm, thickness 2.5–10 mm) and 205 (19%) had large tumours (LBD >16 mm or thickness >10 mm).
The ciliary body was involved in 148 (13.7%) cases and extraocular extension was noted in 20 (1.8%). TNM staging data are shown in Table 1. Patients were followed up for a median of 44 months (range 12–1396 months).
On further analysis of patient subgroups from each referral centre, the tumour characteristics varied considerably (Table 2). The median diameter and thickness of tumours treated with PBR in Glasgow were larger than that of other centres, with a higher proportion of ciliary body involving lesions (27%). In contrast, the median and mean distance of tumours from the disc and fovea referred from London were similarly low, suggesting a preponderance of posteriorly located small to medium tumours referred for PBR (only 1.5% involving the ciliary body, and none were in the large tumour category). Liverpool figures suggested a mixture of cases, with 14% involving the ciliary body, many tumours close to the disc (median distance to disc 0.9 mm) but a mean distance of 4.2 mm; Liverpool also treated a high proportion of small tumours (51% of their cases).
Statistical analysis with a single-factor ANOVA showed a significant difference between the referred tumour thickness and largest basal diameter between Glasgow and the other three centres (P < 0.001) with higher values for each. The differences between the remaining three centres were not statistically significant. The variance in referral parameters was lowest in the London cohort (4.16 thickness, 7.62 diameter) and highest in the Liverpool cohort (18.18 thickness, 42.10 diameter).
The data for distance of tumour to disc and fovea was more incomplete. The differences between all centres were statistically different for distance to the optic disc centre except between Liverpool and Glasgow (P = 0.22); however, both Liverpool and Glasgow demonstrated the greatest variance (36.0, 24.2) in comparison to London with the lowest variance of 1.88. On the other hand, the distance to the fovea was not significantly different between the centres.
The common complications following proton therapy are shown in Table 3. Tumour recurrence was uncommon, with successful tumour control in 82–93%. Significant side effects eventually lead to secondary enucleation that ranged from 5 to 33%; the variability is likely due to the differences in the tumour phenotypes treated.
Discussion
Proton beam therapy is an effective treatment for UM; local tumour control rates as well as eye preservation in UM are very high at >90% for the period of the study, comparable to brachytherapy [16,17,18,19,20,21]. Complications include radiation maculopathy, optic neuropathy and toxic tumour syndrome. These complications are in the majority related to the size of the tumour as well the proximity of the tumour to visually sensitive structures such as the fovea and optic nerve head [19].
The availability of proton treatment varies across Europe and the world. As such, the indications for treatment are dependent on individual surgeon choice, the ability to cover the cost of travel and treatment, and accessibility. Some centres use protons predominantly for lesions considered too large for radio-plaque brachytherapy as it is more readily available in many centres [17]. Concurrently, the treatment of small posteriorly located tumours is more successful with proton treatment [5, 22,23,24,25], with a slower and more gradual loss of vision, than with subfoveal or notched plaques, and may offer an alternative indication for proton referral [26].
In the UK, the alternative treatments include ruthenium-106 plaque brachytherapy, stereotactic radiotherapy, surgical resection (enucleation, endo/exo-resection) or photodynamic therapy. The choice of this relatively expensive treatment modality in preference to other interventions is case dependent; these decisions will depend on clinician experience and exposure to these different treatment regimens as well as patient choice of available options. The choice of proton treatment may include those lesions difficult to address with radio-plaque treatment (at the disc, or with diffuse edges for example) or those too large for plaque treatment (generally considered for ruthenium-106 to be 6 mm in thickness). It is clear that the referral criteria for proton beam treatment vary between centres even within the UK, despite the availability to all patients under the NHS. Glasgow refers a significantly higher proportion of large ciliary body tumours, whereas Sheffield and London tend to consider medium sized and more posteriorly located tumours for proton treatment; this may be influenced by the availability of stereotactic radiotherapy in Sheffield for larger tumour subgroups. Liverpool seems to have a combination of both of these groups, with a high proportion of small tumours which may in part be related to the proximity of the proton beam service to the local team with better access, as well as a preponderance to treat smaller tumours early. The rates of complications and secondary enucleations will reflect this variation in the tumour phenotypes treated at these locations. This is the first UK-wide audit enabling the capture of all patients treated at the single proton centre.
Summary
What is known about this topic
-
Proton beam radiotherapy is a successful treatment for uveal melanoma.
What this study adds
-
There is great variation in the indications for treating uveal melanoma with protons. This includes small tumours at the posterior pole, and larger anterior tumours beyond the range for standard plaque brachytherapy.
-
Although this will be surgeon dependent, it will also depend on patient factors such as accessibility and patient choice. This may impact the rates of post-treatment complications, including the need for secondary enucleation.
Data availability
Raw data are available on request.
References
Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14:483–95.
Goossens ME, Van den Bulcke M, Gevaert T, Meheus L, Verellen D, Cosset JM, et al. Is there any benefit to particles over photon radiotherapy? Ecancermedicalscience. 2019;13:982.
Schreuder AN, Shamblin J. Proton therapy delivery: what is needed in the next ten years? Br J Radio. 2020;93:20190359.
Burnet NG, Mackay RI, Smith E, Chadwick AL, Whitfield GA, Thomson DJ, et al. Proton beam therapy: perspectives on the National Health Service England clinical service and research programme. Br J Radio. 2020;93:20190873.
Gragoudas ES, Goitein M, Koehler AM, Verhey L, Tepper J, Suit HD, et al. Proton irradiation of small choroidal malignant melanomas. Am J Ophthalmol. 1977;83:665–73.
Hrbacek J, Mishra KK, Kacperek A, Dendale R, Nauraye C, Auger M, et al. Practice patterns analysis of ocular proton therapy centers: the International OPTIC Survey. Int J Radiat Oncol Biol Phys. 2016;95:336–43.
Verma V, Mehta MP. Clinical outcomes of proton radiotherapy for uveal melanoma. Clin Oncol (R Coll Radio). 2016;28:e17–27.
Wang Z, Nabhan M, Schild SE, Stafford SL, Petersen IA, Foote RL, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2013;86:18–26.
Boldt HC, Binkley E. Treating small choroidal melanoma: smaller is better. JAMA Ophthalmol. 2018;136:1333–4.
Hussain R, Czanner G, Taktak A, Damato B, Praidou A, Heimann H. Mortality of patients with uveal melanoma detected by diabetic retinopathy screening. Retina. 2020;40:2198–2206.
Jouhi S, Jager MJ, de Geus SJR, Desjardins L, Eide NA, Grange JD, et al. The Small Fatal Choroidal Melanoma Study. A survey by the European Ophthalmic Oncology Group. Am J Ophthalmol. 2019;202:100–8.
Toutee A, Angi M, Dureau S, Levy-Gabriel C, Rouic LL, Dendale R, et al. Long-term visual outcomes for small uveal melanoma staged T1 treated by proton beam radiotherapy. Cancers (Basel). 2019;11:1047.
Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63:385–91.
Kacperek A. Protontherapy of eye tumours in the UK: a review of treatment at Clatterbridge. Appl Radiat Isot. 2009;67:378–86.
Sikuade MJ, Salvi S, Rundle PA, Errington DG, Kacperek A, Rennie IG. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye (Lond). 2015;29:1194–8.
Mishra KK, Daftari IK. Proton therapy for the management of uveal melanoma and other ocular tumors. Chin Clin Oncol. 2016;5:50.
Desjardins L, Lumbroso-Le Rouic L, Levy-Gabriel C, Cassoux N, Dendale R, Mazal A, et al. Treatment of uveal melanoma by accelerated proton beam. Dev Ophthalmol. 2012;49:41–57.
Kamran SC, Collier JM, Lane AM, Kim I, Niemierko A, Chen YL, et al. Outcomes of proton therapy for the treatment of uveal metastases. Int J Radiat Oncol Biol Phys. 2014;90:1044–50.
Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62:1405–11.
Egger E, Zografos L, Schalenbourg A, Beati D, Bohringer T, Chamot L, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55:867–80.
Egger E, Zografos L, Munkel G, Bohringer T, Bercher L, Chamot L. Results of proton radiotherapy for uveal melanomas. Front Radiat Ther Oncol. 1997;30:111–22.
Gragoudas ES. 1996 Jules Gonin Lecture of the Retina Research Foundation. Long-term results after proton irradiation of uveal melanomas. Graefes Arch Clin Exp Ophthalmol. 1997;235:265–7.
Desjardins L, Lumbroso-Le Rouic L, Levy-Gabriel C, Dendale R, Delacroix S, Nauraye C, et al. Combined proton beam radiotherapy and transpupillary thermotherapy for large uveal melanomas: a randomized study of 151 patients. Ophthalmic Res. 2006;38:255–60.
Pica A, Weber DC, Vallat L, Bergin C, Hrbacek J, Schweizer C, et al. Good long-term visual outcomes of parapapillary choroidal melanoma patients treated with proton therapy: a comparative study. Int Ophthalmol. 2021;41:441–52.
Thariat J, Grange JD, Mosci C, Rosier L, Maschi C, Lanza F, et al. Visual outcomes of parapapillary uveal melanomas following proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95:328–35.
Hussain R, Heussen FM, Heimann H. OCT changes in peri-tumour normal retina following ruthenium-106 and proton beam radiotherapy for uveal melanoma. Br J Ophthalmol. 2021;105:648–52.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the conceptualisation of this paper, data collection, data analysis and/or write-up of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussain, R.N., Chiu, A., Pittam, B. et al. Proton beam radiotherapy for choroidal and ciliary body melanoma in the UK—national audit of referral patterns of 1084 cases. Eye 37, 1033–1036 (2023). https://doi.org/10.1038/s41433-022-02178-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02178-0