Abstract
Background/Objectives
To study the development, evolution, outcomes, and prognostic factors of lamellar macular hole (LMH) in highly myopic (HM) patients.
Methods
Fifty eyes from 47 HM patients with LMHs were retrospectively enrolled. Relevant pre- and post-LMH optical coherence tomography findings and visual acuity were collected. Structural progression was defined as an increase in the height of retinoschisis, and the development of foveal detachment, full-thickness macular hole, or retinal detachment.
Results
Four traction-related developmental processes were identified. Type 1 LMHs (8, 16%) developed from foveal avulsion caused by vitreomacular traction. Type 2 (32, 64%) and type 3 LMHs (5, 10%) formed from ruptured parafoveal and central foveal cysts, respectively. Progressive foveal thinning caused by epiretinal membranes (ERMs) without cystic changes led to type 4 LMHs (5, 10%). Retinoschisis developed before (9 eyes), after (10 eyes), or simultaneously with (6 eyes) the LMH formation. Structural progression was noted in 50%, 53%, 0%, 100% of patients with type 1–4 LMHs, respectively. Multivariable Cox proportional hazard model showed that greater residual foveal thickness (P = 0.001, adjusted odds ratio = 0.22, 95% confidence interval [CI], 0.08 ~ 0.56), and the absence of retinoschisis were protective against structural progression. Multivariable linear regression showed that poor baseline visual acuity (P < 0.001, β = 0.74, 95% CI 0.41 ~ 1.07) and type 4 LMH predicted worse visual outcomes.
Conclusions
Four traction-related LMH developmental processes were observed in HM eyes and exhibited different evolution and outcomes. LMHs with foveal thinning induced by ERMs had the worst outcomes.
Similar content being viewed by others
Introduction
A lamellar macular hole (LMH) is a vitreomacular disorder characterized by irregular foveal contour [1,2,3]. Previous studies have focused on the treatment strategies and outcomes of LMHs [4,5,6,7,8]. However, the formation pathways of LMHs are still debated. With the advent of optical coherence tomography (OCT), structural changes associated with LMH have been identified, including epiretinal membrane (ERM), epiretinal proliferation (EP), and vitreomacular traction (VMT) [1, 5, 9]. Govetto et al. observed two morphologies of LMHs, and speculated them to be a tractional or a degenerative evolutional process [10]. Recently, Hubschman et al. redefined the LMH-related lesions into “LMH”, “ERM with foveoschisis”, and “macular pseudohole”, and echoed the hypotheses of tractional and degenerative evolution pathways [3].
In highly myopic (HM) eyes, the clinical course of LMHs is more complicated and unstable due to the complex tractions, including the adherent posterior hyaloid, ERM, rigid internal limiting membrane, posterior staphyloma, and the presence of macular retinoschisis [11,12,13,14]. Therefore, the development of LMHs in HM eyes may be distinct from that in non-HM eyes, and worth investigating separately. In the present study, we aimed to elucidate the LMH development processes in HM eyes. Furthermore, we investigated the evolution and outcomes of LMHs with different formation processes, and analysed the prognostic factors that could provide clues to follow-up plans and treatment strategies for LMH in HM eyes.
Materials And methods
Study population
Consecutive cases of HM with LMH with medical records available for the period between January 2010 and May 2020 were retrospectively reviewed. High myopia was defined as a spherical equivalent refractive error <−6.0 dioptres and/or an axial length >26.5 mm. The diagnosis of LMH was made using the following criteria: (1) irregular foveal contour; (2) defects in the inner fovea (with or without actual loss of tissue); (3) separation of the inner and outer retinal layers in the fovea, and (4) absence of a full-thickness foveal defect [9]. Patients with pre- and post- LMH OCT records and had follow-up more than one year after LMH formation were included. Patients with a history of retinal disease other than myopic tractional maculopathy, vascular or inflammatory diseases, major ocular trauma, or intraocular surgery other than cataract surgery were excluded. The study was approved by the Institutional Review Board of the National Taiwan University Hospital and followed the tenets of the Declaration of Helsinki.
Clinical characteristics and ocular examinations
Best-corrected visual acuity (BCVA) was recorded at the initial visit, at the time of LMH formation, and at the time of the last follow-up. Autorefraction (auto kerato-refractometer KR-8800, Topcon Inc., Tokyo, Japan) and axial length (Lenstar LS 900, Haag-Streit, Koeniz, Switzerland) were measured. Every patient underwent OCT (RTVue Model-RT 100 scanner, version 3.5; Optovue Inc., Fremont, CA, USA) at 3- to 6-month intervals. Standard 8 mm or 10 mm horizontal and vertical OCT scans, centred on the fovea, were obtained. The following OCT parameters were documented or measured using the manual calliper function of the built-in software: central retinal thickness (CRT), maximal horizontal and vertical LMH diameters, and minimal residual foveal thickness (RFT). The RFT was measured as the shortest distance from the base of the LMH to the inner layer of Bruch’s membrane at the fovea. The presence of an ERM, EP, macular retinoschisis, VMT, foveal bump, integrity of the ellipsoid zone, and the shape of LMH were recorded. EP was defined as a substantial homogeneous mound with medium reflectivity at the hole edge and on the epiretinal surface by OCT images. V-shaped LMHs had a smaller diameter intraretinally than at the retinal surface in either the horizontal or vertical section, while A-shaped LMHs had a larger diameter intraretinally in both the horizontal and vertical sections. ERM foveoschisis was defined as having a contractile ERM and foveoschisis at the level of the Henle fibre layer confined to the parafoveal region [3]. Only eyes with extensive retinoschisis involving the entire macula were considered as having macular retinoschisis (or simply retinoschisis) [15]. Anatomical progression was defined as retinoschisis progression (an increase in maximal retinoschisis height >150 μm), development of foveal detachment, impending full-thickness macular hole (MH), full-thickness MH, or MH with retinal detachment.
Statistical analysis
All statistical analyses were performed using R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). The clinical characteristics of different LMH developmental types were compared using the Kruskal-Wallis test or chi-squared test. If the P-value of the Kruskal-Wallis test was significant, Dunnett’s post-hoc analysis was performed. The clinical characteristics at different time points were compared using the paired Wilcoxon rank-sum test. Factors associated with final visual acuity were analysed using univariable linear regression. Factors associated with anatomical progression were analysed using univariable Cox proportional hazards regression model. Variables with P < 0.1 in the univariable models were included in the multivariable models. Statistical significance was set at P < 0.05.
Results
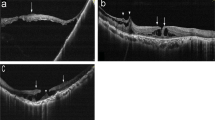
Fifty eyes of from 8 men and 39 women (mean age: 61.0 ± 7.5 years) were enrolled. The mean axial length was 29.42 ± 2.13 mm. The median follow-up time before and after LMH formation was 35 months (range: 5–90 months) and 47 months (range: 13–94 months), respectively. Four types of LMH formation processes were identified, including (1) type 1, LMH developed after foveal avulsion induced by VMT (8 cases) (Fig. 1a, b); (2) type 2, LMH developed after the inner medial wall of a parafoveal cyst or retinoschisis ruptured (32 cases) (Fig. 1c, d); (3) type 3, the ruptured central foveal cysts induced by ERM led to LMH formation (5 cases) (Fig. 1e, f); and (4) type 4, progressive foveal thinning was induced by an ERM, and the LMH formed without an intervening stage of cyst or retinoschisis (5 cases) (Fig. 1g, h). Table 1 shows the clinical characteristics of LMH with different formation processes. Type 2 LMH was the most common (64%). Type 4 LMH had the highest rate (80%) of ellipsoid zone disruption, but no EP. Sixty percent of type 3 LMHs were A-shaped, while V-shaped LMH was the predominant form in other types. In some patients, the LMH initially resembled a “tractional type” LMH and evolved into a “degenerative type” configuration later (Fig. 1i-l). Table 2 shows the changes in the clinical characteristics of LMH during the follow-up. The BCVA of patients with type 2 (P = 0.003, paired Wilcoxon rank-sum test) and type 4 LMHs (P = 0.029, paired Wilcoxon rank-sum test) decreased significantly during follow-up. Among all types, patients with type 4 LMH had the most significant decline (1.03 ± 0.69) of visual acuity (P = 0.024, Kruskal-Wallis test). The vertical depth of type 2 (P = 0.041, paired Wilcoxon rank-sum test) and type 4 (P = 0.017) LMHs increased significantly. The type 4 LMHs had the more significant increase in vertical depth than the type 3 LMHs (P = 0.032, post-hoc analysis of Kruskal-Wallis test). A significant widening of the LMH was seen in type 3 LMHs (P = 0.001, paired Wilcoxon rank-sum test). The RFT of type 2 (P = 0.040, paired Wilcoxon rank-sum test) and type 4 LMHs (P = 0.024) decreased significantly. The type 4 LMHs had the more significant decrease in RFT than the type 1 LMHs (P = 0.015, post-hoc analysis of Kruskal-Wallis test).
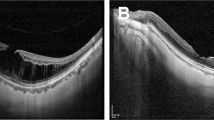
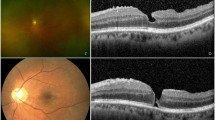
a–h Four types of developmental processes of LMH (a, b) Type 1 LMH develops after the avulsion of foveal tissue induced by vitreomacular traction (c, d) Type 2 LMH develops after the disruption of the medial wall of the parafoveal cyst (e, f) Type 3 LMH develops after the deroofing of the central foveal cyst (g, h) Type 4 LMH develops from the progressive central foveal thinning induced by the epiretinal membrane. Intraretinal cyst or schisis is not observed. i–l A representative case of LMH evolved from the “tractional configuration” into “degenerative configuration”. i, j LMH develops from the disruption of the foveal cyst by vitreomacular traction (type 1). k It has the features of “tractional LMH” with foveoschisis and a sharp edge. l Four years later, it has transformed into a “degenerative LMH” with a foveal bump and a round edge (asterisk). m–r The different chronological sequences of the LMH and macular retinoschisis (m, n) Group 1: LMH formed before the development of retinoschisis (o, p) Group 2: LMH and retinoschisis developed concurrently (q, r) Group 3: LMH developed in the presence of retinoschisis.
Half of the patients had macular retinoschisis. According to the chronological sequence of the development of LMH and macular retinoschisis, patients could be categorized into three groups: (1) group 1 (10 eyes), in which the LMH developed first and transformed into LMH with retinoschisis (Fig. 1m, n); (2) group 2 (6 eyes), in which the LMH and retinoschisis developed at the same time (Fig. 1o, p); (3) group 3 (9 eyes), in which the retinoschisis developed first, and the LMH developed later (Fig. 1q, r). Table 1 shows the clinical characteristics of the three groups (group 1–3) and the eyes without retinoschisis (group 0). Fifty percent and 66% of patients with type 1 and 2 LMHs developed retinoschisis, respectively. None of the type 3 or type 4 LMHs were associated with retinoschisis. Eyes in group 2 had longer axial lengths than eyes in group 0 or 1 (P = 0.043). Other clinical characteristics were not significantly different between the groups. In Table 2, patients in group 1 had a significant decrease in visual acuity (P = 0.047) and RFT (P = 0.002), and an increase in vertical extension (P = 0.045) during the follow-up. The between-group comparison of changes in clinical features were all non-significant.
Table 3 shows the outcomes of LMH with different developmental processes. The number of eyes with anatomical progression was 4 (50%), 17 (53%), 0 (0%), and 5 (100%) in types 1, 2, 3, and 4, respectively (P = 0.015, chi-squared test). All eyes with type 4 LMH eventually developed full-thickness MH, while none of the eyes with type 3 LMH had anatomical progression. Only 24% of eyes without retinoschisis had anatomical progression, while nearly all eyes in group 1 and 2 had anatomical progression.
Table 4 shows the factors associated with anatomical progression using Cox proportional hazards regression analysis. Worse visual acuity at the time of LMH formation, more ellipsoid zone disruption, and group 1 or 2 LMHs were associated with anatomical progression, while greater RFT and the presence of EP were protective factors. The multivariable model showed that greater RFT remained a protective factor (P = 0.001, odds ratio = 0.22), while eyes with retinoschisis had a higher risk of anatomical progression.
Table 4 shows the factors associated with visual outcomes. Univariable linear regression showed that age, sex, visual acuity at the time of LMH formation, RFT, the presence of ellipsoid zone disruption and EP, and types of LMH were associated with final visual acuity. Multivariable linear regression showed that visual acuity at the time of LMH formation remained positively associated with final visual acuity (P < 0.001, β = 0.74). Type 1–3 LMHs were associated with better final visual acuity than type 4 LMHs.
Discussion
Although idiopathic LMH has been investigated extensively, most studies have focused on the stages after LMH formation [4,5,6,7,8, 13, 14, 16], with only a few studies examining the structural changes both before and after LMH formation. With the multi-layered, complicated traction on the weakened foveal tissue, the development and evolution of LMH in HM eyes may be more complicated than those in non-HM eyes. However, the discussion on LMH in myopic eyes is even less in the literature. In this study, the structural changes before and after LMH formation in eyes with HM were specifically studied to determine the formation and evolution of this specific entity. These results may also have clinical implications in idiopathic LMH formation.
Highly myopic eyes are more susceptible to abnormal posterior vitreous detachment and ERMs [17,18,19]. The posterior hyaloid firmly adheres to the retinal surface, and is connected to the vitreous cortex. ERM can fuse with this membrane or exist between this posterior hyaloid membrane and the retinal surface [5]. The presence of retinoschisis also changes the foveal structure, traction force, and its direction in HM eyes. In the present study, we observed four types of LMH developmental processes. In short, type 1 LMHs started from foveal tissue avulsion caused by abnormal posterior vitreous detachment and mimicked the abortive development of full-thickness MH; type 2 and type 3 LMHs originated from ruptured parafoveal cysts/schisis and central foveal cysts, respectively. Type 4 LMHs were induced by the persistent ERM traction causing progressive central foveal thinning without going through the parafoveal or foveal cyst stages. In the pre-LMH and the early LMH stages, tractional forces from ERM or vitreous adhesion could be identified in all cases, suggesting that traction, instead of degeneration, was the primary mechanism of LMH formation in eyes with HM. Since macular retinoschisis is a common clinical feature in HM, we specifically examined the chronological sequence of LMH formation and the development of retinoschisis. Retinoschisis could develop before, after, or simultaneously with the LMH formation.
We found that LMHs had distinct evolutions and outcomes according to the different developmental processes, and our classification might have some clinical relevance. Patients with the most common type 2 LMH had ERM with foveoschisis or myopic retinoschisis as a pre-LMH condition. Subsequently, the medial walls of parafoveal cysts ruptured and transformed into LMHs. When combined with macular retinoschisis, LMHs deepened, and the RFT decreased during follow-up, accompanied by visual deterioration. Half of the patients experienced anatomical worsening or progression. Type 3 LMHs developed from the deroofing of foveal cysts induced by vitreous traction or ERM. Subsequently, foveoschisis between the outer plexiform layer and the outer nuclear layer might have occurred, and the horizontal width of LMHs increased significantly. Therefore, type 3 LMHs often acquired an A-shaped configuration, which was protective against progression [14]. We postulated the wider base of A-shaped LMH could dissipate the traction force, thus visual function was more likely to be preserved in this type. Type 1 LMH formed by avulsion of the foveal tissue owing to VMT, which was regarded as the abortive form of full-thickness MH. The width of LMHs and RFT remained stable since the traction had been released. In types 1–3 LMHs, avulsion of foveal tissue and disruption of cysts or schistic cavities were accompanied by the partial reduction of traction. In contrast, type 4 LMHs were induced by ERM without an intraretinal cyst stage. The persistent ERM traction acted directly on the fovea, and caused foveal thinning. Hence, the vertical depth of the LMH increased, and the residual foveal tissue became thinner. This type of LMH was prone to visual deterioration, and all progressed to full-thickness MHs.
LMHs without retinoschisis tended to be stable, except for type 4 LMHs. In contrast, our previous study showed that half of the LMH with retinoschisis developed MH or foveal detachment [14]. The persistent traction from retinoschisis may contribute to the progression of LMH. Rino et al. showed that LMHs with retinoschisis were associated with worse visual acuity [6]. In the present study, group 1 LMHs tended to deepen and be associated with visual deterioration. The LMH was a weak point in the foveal structure, and the tractional force from newly developed retinoschisis caused further enlargement of the LMH and thinning of the outer floor. Since most group 3 LMHs developed from the disruption of the pre-existing schistic tissue, they usually remained stable unless the macular retinoschisis progressed.
We further investigated the factors predicting visual outcomes and anatomical progression. Thirty percent of LMHs were associated with EP, and were characterized by a wider defect, more ellipsoid zone disruption, and poor visual outcome [3, 7, 10, 20, 21]. Despite its controversial origin, EP was considered a Müller-cell-driven healing response to the retinal defect [3, 21, 22]. Epiretinal proliferation was more frequently found in LMHs (40–60%) in HM eyes, were more widespread due to larger retinal defects [5, 6], and might have different clinical characteristics and behaviours. Lai et al. found that EP in HM eyes did not affect visual outcomes [5]. In the present study, EP were observed in 40–60% of the type 1–3 LMHs. Type 4 LMHs were spared from EP but had the worst outcomes. We speculated that EP may be a repair process of the structural defect, or even act as a cushion against tractional force [14]. Eyes with persistent traction that do not develop EP may be vulnerable to progression. Moreover, LMHs with thinner RFT had higher risk of progression. Our previous study showed that the wide base and thicker residual fovea of A-shaped LMH were protective against anatomical progression [14]. In contrast, V-shaped LMHs were unstable, since the tractional force was concentrated and acted directly on their small floor [14]. In this study, a protective effect of A-shaped LMH was not found, but that of a thicker residual foveal floor was observed. The smaller number of cases and shorter follow-up time may explain the difference. The presence of macular retinoschisis, which indicated a persistent traction force on the macular area [23], together with the thinner residual foveal floor, was found to predispose to anatomical progression. However, visual outcomes are not inevitably worse in LMHs with macular retinoschisis. Contrarily, eyes with worse BCVA at the time of LMH formation or type 4 LMHs tended to have poor final BCVA. LMHs exhibiting these features should have closer follow-up to prompt timely surgical intervention.
Recently, Parolini et al. proposed a new myopic traction maculopathy staging system including those with LMH [23]. In their staging system, tangential traction induced LMH (stage 1b) in the presence of inner and/or outer retinoschisis (stage 1a) [23]. The observation in the present study; however, showed that in those with retinoschisis, 40% of them had LMH before the development of retinoschisis. Further, our study showed that LMHs in HM eyes could develop due to tangential, vertical, or even oblique traction. In type 1 LMHs, the vertical traction from the vitreous adhesion caused foveal tissue avulsion. In type 2 LMHs, the persistent vertical traction caused the progression of retinoschisis and disruption of the schistic column. Type 4 LMHs may be resulted from the tangential traction from ERM. We thought that the tractional force in HM eyes was too complicated to indicate that a certain clinical feature was contributed to the force from single direction. Lastly, not all of the eyes had progression by the proposed stage. For example, those with outer retinoschisis (stage 2a) might downgrade to stage 1b with the LMH formation possibly because the traction was partially released. Therefore, we believed that the present study may provide additional information regarding the development of LMH in HM eyes.
The observation of the development and evolution of LMHs in HM eyes may have implications in the formation of idiopathic LMHs, of which the classification and definition have been revised several times [2, 3, 10]. Govetto et al. classified this macular lesion into “tractional LMH” and “degenerative LMH” [10]. Hubschman et al. further redefined the LMH-related lesions into “ERM with foveoschisis”, “LMH”, and “macular pseudohole”[3]. “ERM with foveoschisis” corresponds to the “tractional LMH”. The newly-defined “LMH”, comparable to the “degenerative LMH” has round-edged intraretinal cavitation affecting all retinal layers, and is associated with EP [10]. The classification implies the two different pathways of LMH development. However, recent studies have shown that tractional forces play a primary pathogenic role in degenerative LMHs [24, 25]. The VMT or ERM disrupts the Müller cells cone and induces outer retinoschisis. The damage of Henle fibres is followed by the degeneration of photoreceptors and the inner nuclear layer, which results in the formation of degenerative LMH [24]. In our cohort of HM eyes, the tractional forces could be identified in all cases. Different types of tractional forces, their points of exertion, and the net traction direction could lead to distinct formative and evolutionary processes. Clinical features of degenerative type LMH would develop later (Fig. 1i–l). Whether this observation could be applied to idiopathic conditions remains to be determined.
This study has several limitations. It is difficult to collect a large cohort with comprehensive records of LMH development, especially for type 3 and 4 LMHs. Although a prospective study may be ideal, it may be difficult to set a time limitation. We performed OCT in regular interval, but some evolutionary changes might have been missed. However, this is the first report on the developmental processes of LMH in patients with HM, and the clinical characteristics and outcomes were significantly different between the types. Therefore, the developmental processes of LMHs in HM eyes proposed in the present study have clinical values.
In conclusion, we presented four different developmental processes of LMHs in HM eyes. Tractional force, either vitreoretinal traction or ERM, could be identified during the development of every LMH. LMHs with different developmental processes have distinct clinical characteristics and functional and anatomical outcomes. Type 3 LMHs were at lower risk of anatomical progression, whereas type 4 LMHs were susceptible to visual deterioration and progression into full-thickness MHs. LMHs with retinoschisis had a more unstable clinical course than those without retinoschisis. Eyes with thinner RFT and poor visual acuity at the time of LMH formation had worse outcomes. Frequent follow-up and timely intervention are warranted in LMHs with a higher risk.
Summary
What was known before
-
Idiopathic lamellar macular hole may exhibit a tractional or a degenerative configuration.
-
The staging system of myopic traction maculopathy including lamellar macular hole had been proposed. However, the formation process of lamellar macular hole in highly myopic eyes had not been fully elucidated.
What this study adds
-
Lamellar macular hole in highly myopic eyes may develop through different formation processes and all of them have the involvement of traction. Degenerative configuration may occur in some stages during the evolution process.
-
Lamellar macular hole in highly myopic eyes formed by epiretinal membrane induced progressive foveal thinning in the absence of an intervening stage of cyst or retinoschisis tended to progress into full-thickness macular hole and had the worst visual outcome.
-
Lamellar macular hole with retinoschisis were more susceptible to anatomical progression than those with simple lamellar macular hole in highly myopic eyes. Lamellar macular hole may develop before, after, or simultaneously with the retinoschisis.
Data availability
The data are available upon reasonable request.
References
Frisina R, Pilotto E, Midena E. Lamellar macular hole: State of the art. Ophthalmic Res. 2019;61:73–82.
Witkin AJ, Ko TH, Fujimoto JG, Schuman JS, Baumal CR, Rogers AH, et al. Redefining lamellar holes and the vitreomacular interface: An ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–97.
Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104:1741–7.
Tanaka Y, Shimada N, Moriyama M, Hayashi K, Yoshida T, Tokoro T, et al. Natural history of lamellar macular holes in highly myopic eyes. Am J Ophthalmol. 2011;152:96–99.e91.
Lai TT, Yang CM. Lamellar hole-associated epiretinal proliferation in lamellar macular hole and full-thickness macular hole in high myopia. Retina. 2018;38:1316–23.
Rino F, Elena Z, Ivan M, Paolo B, Barbara P, Federica R. Lamellar macular hole in high myopic eyes with posterior staphyloma: Morphological and functional characteristics. Graefes Arch Clin Exp Ophthalmol. 2016;254:2141–50.
Haritoglou C, Tadayoni R, Hubschman JP. Lamellar macular hole surgery - current concepts, future prospects. Clin Ophthalmol. 2019;13:143–6.
Danielescu C, Stanca HT, Balta F. The management of lamellar macular holes: A review. J Ophthalmol. 2020. https://doi.org/10.1155/2020/3526316.
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9.
Govetto A, Dacquay Y, Farajzadeh M, Platner E, Hirabayashi K, Hosseini H, et al. Lamellar macular hole: Two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109.
Hattori K, Kataoka K, Takeuchi J, Ito Y, Terasaki H. Predictive factors of surgical outcomes in vitrectomy for myopic traction maculopathy. Retina. 2018;38:s23–s30.
Lin CW, Ho TC, Yang CM. The development and evolution of full thickness macular hole in highly myopic eyes. Eye (Lond). 2015;29:388–96.
dell’Omo R, Virgili G, Bottoni F, Parolini B, De Turris S, Di, et al. Lamellar macular holes in the eyes with pathological myopia. Graefes Arch Clin Exp Ophthalmol. 2018;256:1281–90.
Hsia Y, Ho TC, Yang CH, Hsieh YT, Lai TT, Yang CM. Clinical characteristics and long-term evolution of lamellar macular hole in high myopia. PLoS One. 2020;15:e0232852.
Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156:948–.e941.
Bottoni F, Deiro AP, Giani A, Orini C, Cigada M, Staurenghi G. The natural history of lamellar macular holes: A spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013;251:467–75.
Takahashi H, Tanaka N, Shinohara K, Yokoi T, Yoshida T, Uramoto K, et al. Ultra-widefield optical coherence tomographic imaging of posterior vitreous in eyes with high myopia. Am J Ophthalmol. 2019;206:102–12.
Hayashi K, Manabe SI, Hirata A, Yoshimura K. Posterior vitreous detachment in highly myopic patients. Invest Ophthalmol Vis Sci. 2020;61:33.
Koh V, Cheung CY, Wong WL, Cheung CM, Wang JJ, Mitchell P, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012;53:1018–22.
Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35:720–6.
Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: A distinct clinical entity. Retina. 2014;34:1513–23.
Pang CE, Maberley DA, Freund KB, White VA, Rasmussen S, To E, et al. Lamellar hole-associated epiretinal proliferation: A clinicopathologic correlation. Retina. 2016;36:1408–12.
Parolini B, Palmieri M, Finzi A, Besozzi G, Lucente A, Nava U. et al. The new myopic traction maculopathy staging system. Eur J Ophthalmol.2020. https://doi.org/10.1177/1120672120930590.
Bringmann A, Unterlauft JD, Wiedemann R, Barth T, Rehak M, Wiedemann P. Degenerative lamellar macular holes: Tractional development and morphological alterations. Int Ophthalmol. 2021;41:1203–21.
Compera D, Cereda MG, Schumann RG, Bottoni F. Development and progression of a lamellar macular hole with lamellar hole-associated epiretinal proliferation. Retin Cases Brief Rep. 2019;13:371–5.
Author information
Authors and Affiliations
Contributions
Design and implantation of the study (YH, CYL, CMY); Collection of the data (YH, CYL, TCH, CHY, CMY); Collection, management, analysis, and interpretation of the data (YH, CYL, CMY); Drafting of the manuscript (YH); Review, revise, and approval of the manuscript (YH, CYL, TCH, CHY, CMY).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hsia, Y., Lee, CY., Ho, TC. et al. The development and evolution of lamellar macular hole in highly myopic eyes. Eye 37, 1170–1177 (2023). https://doi.org/10.1038/s41433-022-02086-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02086-3
This article is cited by
-
Lamellar macular hole in highly myopic eyes and insights into its development, evolution, and treatment: a mini-review
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Vitreoretinal Interface Changes After Anti-vascular Endothelial Growth Factor Treatment in Highly Myopic Eyes: A Real-World Study
Ophthalmology and Therapy (2023)
-
Correction: Formation and evolution of idiopathic lamellar macular hole-a pilot study
BMC Ophthalmology (2022)
-
Formation and evolution of idiopathic lamellar macular hole-a pilot study
BMC Ophthalmology (2022)