Abstract
Purpose
To investigate predictors for macular atrophy (MA) involving the fovea after photodynamic therapy (PDT) followed by pro re nata (PRN) treatment for polypoidal choroidal vasculopathy (PCV).
Methods
This prospective observational study analysed treatment-naïve eyes with symptomatic PCV without MA at baseline which were followed up for 5 years. All eyes were initially treated with PDT, followed by a PRN regimen of anti-vascular endothelial growth factor (VEGF) therapy and/or PDT. We assigned eyes with and eyes without development of MA involving the fovea 5 years after the initial treatment into MA and non-MA groups, respectively. Baseline parameters and the number of treatments were compared between the two groups.
Results
Seventy-two eyes of 69 consecutive patients were included, and 29 eyes of 29 patients were analysed. Twelve (41%) and 17 (59%) eyes were assigned into the MA and non-MA groups, respectively. There were significant differences in subfoveal choroidal thickness (226.2 ± 47.8 μm vs. 278.8 ± 68.1 μm, P = 0.03) and number of anti-VEGF injections (13.7 ± 9.6 vs. 5.4 ± 5.6, P = 0.007) between the MA and non-MA groups, but not in the number of PDT sessions (P = 0.71). Best-corrected visual acuity at 5 years in the MA group was lower than in the non-MA group (P = 0.048).
Conclusion
Our long-term observation suggests that a thin subfoveal choroid at baseline and many followed anti-VEGF injections in a PRN regimen increase the risk for development of MA involving the fovea 5 years after PDT.
Similar content being viewed by others
Introduction
Polypoidal choroidal vasculopathy (PCV) is a clinical subtype of neovascular age-related macular degeneration (AMD) characterised by branching vascular networks terminating in polypoidal lesions [1]. Some pathological studies have shown that vascular endothelial growth factor (VEGF) is involved in the development of PCV as well as AMD [2, 3]. Therefore, like AMD, PCV is treated by intravitreal injections of anti-VEGF agents such as ranibizumab and aflibercept, and their effectiveness has been recognised [4,5,6]. However, the eyes often show recurrent exudates, necessitating continuous injections.
Photodynamic therapy (PDT) is highly effective for regression of polypoidal lesions and for resolution of the associated exudates [7, 8]. Recently, a combination of anti-VEGF and PDT for PCV was reported to be effective for improving vision and reducing the need for additional injections [9, 10]. However, several studies have reported that PDT damages the choriocapillaris and induces vaso-occlusive effects not only on choroidal neovasculopathy but also on the normal vasculature of the choriocapillaris and choroid [11, 12]. Thus, PDT can induce retinal pigment epithelium (RPE) atrophy above the choriocapillaris and choroid at the macula, which is referred to as macular atrophy (MA) when it develops over a prolonged period of time.
A previous study reported that MA involving the fovea tended to develop more frequently in eyes with PCV that were treated with PDT combined with intravitreal ranibizumab injection (IVR) than in those treated with IVR alone over a 5-year follow-up period (45.0% vs. 24.3%, P = 0.06). That study also showed that the 5-year best-corrected visual acuity (BCVA) was correlated with the 5-year incidence of MA in eyes treated with PDT combined with IVR [13]. Another recent 2-year follow-up study demonstrated that subfoveal choroidal thinning at baseline is a significant risk factor for the development of MA after anti-VEGF monotherapy in eyes with PCV [14]. However, there is no long-term observational study concerning risk factors for development of MA in PCV after receiving PDT.
The present study assessed the prevalence of MA development after PDT followed by pro re nata (PRN) treatment of anti-VEGF and PDT, and investigated predictors for development of MA in treatment-naïve eyes with PCV over a 5-year follow-up period.
Materials and methods
This prospective observational study was approved by the ethics committee of Kyoto University Graduate School of Medicine (Kyoto, Japan). All study protocols adhered to the tenets of the Declaration of Helsinki. The nature of the study and the possible risks and benefits of participation were explained to all study candidates, who agreed to participate after providing written informed consent.
Participants
This study included treatment-naïve eyes of consecutive patients with symptomatic PCV who received PDT with or without IVR (Lucentis, Novartis, Buläch, Switzerland) initially between January 2008 and March 2015 at Kyoto University Hospital. The patients underwent additional treatment with a PRN regimen of PDT monotherapy, anti-VEGF monotherapy with ranibizumab or aflibercept (Eylea: Bayer, Basel, Switzerland), or a combination of PDT and anti-VEGF therapy. Retinal specialists individually judged the necessity for additional treatment and decided the treatment method.
The inclusion criteria of this study were presence of exudative features involving the macula at baseline. The exclusion criteria were MA at baseline; the presence or history of other eye diseases involving the macula, including retinal vein occlusion, or epiretinal membrane; or severe macular abnormality precluding assessment of the MA, including severe macular fibrosis or large pigment epithelial detachment. We excluded patients who were lost to follow-up for any reason within 5 years from analysis.
All patients underwent a comprehensive ophthalmic examination before treatment, which included measurement of the BCVA using a Landolt C chart and axial length; colour fundus photography; spectral-domain optical coherence tomography (SD-OCT); and fundus fluorescein angiography (FA) and indocyanine green angiography (ICGA). The diagnosis of PCV was based on a branching vascular network terminating in polypoidal swelling, which was detected by retinal specialists on ICGA images. The greatest linear dimension (GLD) was measured on FA images using the built-in software. The incidence of macular exudate status of wet/dry, defined as the presence/absence of subretinal/intraretinal exudate at the macula on basis of OCT images, was investigated 5 years after the initial treatment.
Measurement of retinal and choroidal thickness values
Central retinal thickness, subfoveal RPE thickness, subfoveal choroidal thickness, and subfoveal PED height were manually measured on SD-OCT images using the built-in tool. The averaged distance acquired from horizontal and vertical scans through the fovea was used for analysis. Central retinal thickness was defined as the distance between the vitreoretinal surface and the inner surface of the RPE. Subfoveal RPE thickness was defined as the distance between the inner and outer surfaces of the RPE. Subfoveal choroidal thickness was defined as the distance between the outer surface of Bruch’s membrane and the chorioscleral interface. Subfoveal PED height was defined as the distance between the outer surface of the RPE and the inner surface of Bruch’s membrane in eyes with PED in the subfovea. In eyes without PED in the subfovea, the subfoveal PED height was defined as 0.
Confirmation of macular atrophy involving the fovea
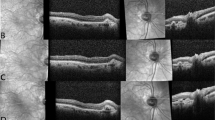
Two investigators (KK and MM) diagnosed MA involving the fovea on the basis of colour fundus photographs and SD-OCT images at baseline and 5 years after treatment, as previously reported (Fig. 1) [13, 15]. Briefly, the criteria were thinning of the RPE band on OCT images, increased signal transmission in the choroid on OCT images, and no contradicting findings on colour fundus photographs. Furthermore, hyper-refractivity on infrared fundus photography served as an additional point of reference in the present study. In cases involving a disagreement between the two graders, the results were discussed until consensus was reached. After eyes with MA involving the fovea at baseline were excluded, the eyes with or without MA involving the fovea at 5 years were assigned into the MA or non-MA groups, respectively.
A, B, E, F, I, K, L, O Images of an eye with symptomatic polypoidal choroidal vasculopathy (PCV) of a man in his 70 s. Colour fundus photographs (A, K), infrared fundus photographs (B, L), fluorescein angiography (FA) (E), Indocyanine green angiography (ICGA) (F), and spectral-domain optical coherence tomography (SD-OCT) images (I, O) acquired at baseline (A, B, E, F, I) and 5 years (K, L, O) after initial photodynamic therapy (PDT) and the subsequent three rounds of PDT and 10 intravitreal injections of ranibizumab. SD-OCT (I, O) corresponds to the location indicated by the green arrow in infrared images (B, L, respectively). MA involving the fovea developed at 5 years. ICGA at baseline (F) shows polypoidal lesions (red arrowhead). The 5-year colour fundus photograph (K) shows hypopigmentation and visible choroidal vessels at the macula, and the 5-year infrared fundus photograph (L) shows hyper-refractivity in the corresponding area (yellow arrowhead). The 5-year SD-OCT image (O) shows highly reflective choroidal signals because of retinal thinning and hypopigmentation of the retinal pigment epithelium (RPE). C, D, G, H, J, K, M, N, P Images of an eye with symptomatic PCV of a woman in her 70 s. Colour fundus photographs (C, M), infrared fundus photographs (D, N), FA (G), ICGA (H), and SD-OCT images (J, P) acquired at baseline (C, D, G, H, J) and 5 years (M, N, P) after the initial combination therapy of PDT and intravitreal injection of ranibizumab and subsequent three rounds of combination therapy. SD-OCT (J and P) corresponds to the location indicated by the green arrow in infrared images (D, N, respectively). ICGA at baseline (H) shows a polypoidal lesion (red arrowhead). In this case, there are no findings indicating the presence of MA at 5 years (I, J, L).
Statistical analysis
All values are presented as means ± standard deviations or as number. The BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analyses. Shapiro–Wilk’s tests were conducted to confirm the normality of the distribution, and Levene’s tests were used to evaluate the equality of variances between the groups. Unpaired t-tests or Mann–Whitney U tests were performed to compare continuous variables with or without normal distribution between the MA and non-MA groups, respectively. The paired t-test was used to compare changes in logMAR BCVA. Fisher’s exact tests were used to compare sets of discontinuous, quantal data between the two groups. All statistical analyses were performed using SPSS version 24 software (IBM, Armonk, New York, USA). Statistical significance was defined as P < 0.05.
Results
In total, 72 eyes of 69 consecutive patients met the inclusion criteria. However, 2, 3 and 9 eyes met the exclusion criteria because of MA at baseline, other eye diseases, and severe macular abnormality precluding assessment of the MA, respectively. Twenty-nine eyes were excluded because of loss to follow-up within 5 years. Thus, 29 eyes of 29 patients (mean age, 75.0 ± 5.1 years, 10 women) were analysed (Fig. 2). MA involving the fovea newly developed during the 5-year observation period in 12 (41%) of the 29 eyes. Thus, the MA and non-MA groups included 12 and 17 eyes, respectively.
Among the baseline parameters assessed in this study, only the subfoveal choroidal thickness showed a significant difference between the MA and non-MA groups (226.2 ± 47.8 μm vs. 278.8 ± 68.1 μm, P = 0.03, Table 1; the odds ratios derived from the logistic regression analysis are shown in Supplementary table 1). No significant intergroup differences were observed in most parameters at baseline, including sex, age, logMAR BCVA, axial length, central retinal thickness, subfoveal RPE thickness, subfoveal PED height, the rate of subretinal fluid at the subfovea, the rate of intraretinal fluid at the fovea, the rate of subfoveal PED, and GLD. Among treatment-associated parameters, the number of anti-VEGF agent injections was greater in the MA group than the non-MA group (13.7 ± 9.6 vs. 5.4 ± 5.6, P = 0.008) but the number of PDT sessions was not different between the groups (P = 0.56, Table 2). Among 5-year parameters, 5-year BCVA was worse in the MA group than in the non-MA group (P = 0.048) but the rate of dry macula did not differ (P = 1.00).
In both groups, BCVA was not significantly different from baseline during the 5-year observation period (P = 0.20 and 0.89, respectively, Fig. 3). BCVA at baseline and at 1, 2, 3 and 4 years were not significantly different between the two groups (P = 0.30, 0.58, 0.37, 0.72 and 0.09, respectively); however, the 5-year BCVA in the MA group was significantly worse than that in the non-MA group (P = 0.048).
At baseline, there is no significant difference between the MA and non-MA groups. The 5-year BCVA is not significantly different from the baseline BCVA in both MA and non-MA groups. However, the 5-year BCVA in the non-MA group is better than the 5-year BCVA in the MA group (*P = 0.048). logMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; MA group, eyes with macular atrophy involving the fovea at 5 years; non-MA group, eyes without macular atrophy involving the fovea at 5 years.
Discussion
The incidence of MA following anti-VEGF therapy tended to be lower in PCV eyes than in the other subtypes of neovascular AMD (typical AMD and retinal angiomatous proliferation) [16,17,18]. Kuroda et al. showed that MA developed in 1.1% and 8.2% of PCV eyes at 12 months after ranibizumab treatment and aflibercept treatment, respectively [16, 17], while Hikichi et al. reported that 16% of PCV eyes developed MA over 3 years of ranibizumab therapy [19]. In the present study, 41% of the analysed eyes developed MA at 5 years. The extended observation period and the use of PDT as the initial treatment may have been responsible for the greater prevalence in the present study.
The subfoveal choroid at baseline was thinner in the MA group than in the non-MA group. Kuroda et al. reported that a thinner subfoveal choroid at baseline was associated with the development of MA 12 months after aflibercept treatment in eyes with neovascular AMD [16]. Choroidal thickness in eyes with PCV is greater than those in the eyes of normal participants and eyes with other subtypes of neovascular AMD [20, 21]. Recently, Cho et al. reported that subfoveal choroidal thinning at baseline is associated with an increased risk of MA 2 years after anti-VEGF monotherapy even in eyes with PCV with thick choroid [14]. The point of performing PDT or not is different between the present study and those previous studies but our results and the results of those previous reports are similar in the point that thin choroid is high risk for MA development.
More followed intravitreal anti-VEGF agent injections were required in the MA group than in the non-MA group. VEGF negatively affects CNV activity; whereas, it plays a critical role in the survival and maintenance of RPE integrity [22]. Therefore, prolonged administration of multiple anti-VEGF injections may have undesirable off-target effects on RPE. Indeed, several studies have reported MA development during anti-VEGF treatment [23, 24]. The HARBOR study showed that monthly treatment trended towards a greater association with MA compared with PRN treatment [23]. However, to date, it is not clear whether the anti-VEGF treatment itself causes MA development [25]. The SEVEN-UP study suggested that MA development during treatment with an anti-VEGF agent could arise secondary to exudation; mechanical damage from angiogenic vessel outgrowth and contraction; and ischemic and inflammatory effects, rather than from the direct toxicity of anti-VEGF therapy [26]. Because anti-VEGF therapy was performed with a PRN regimen in the present study, the eyes that received more frequent injections would have been exposed to exudation more frequently. Our findings did not contradict the results of the previous study.
Short-term choriocapillaris occlusion has been reported to occur after PDT [27, 28]. This finding suggested that PDT could cause MA which is induced with RPE atrophy because the choriocapillaris provides nutrients and oxygen to the RPE [29]. However, there were no significant differences in the number of PDT sessions between the two groups 5 years after the initial PDT in the present study (P = 0.71). Since the blood flow of choriocapillaris is restored due to overexpression of VEGF and its receptors in one month after short-term occlusion [28]. PDT may not cause long-term RPE damage. Furthermore, it may be important for MA development involving the fovea whether PDT exposure area includes the fovea. In the present study, the laser exposure area did not involve the fovea in only three eyes; therefore, it was impossible to verify whether laser exposure to the fovea influenced the development of MA due to relatively small sample size. Further studies to investigate the relationship between the PDT exposure area and MA lesions in larger sample size are necessary to assess this aspect.
Several recent studies reported the visual outcomes 5 years after initial treatment for PCV [13, 30, 31]. Miyamoto et al. reported that, BCVA was maintained for 5 years after the initial treatment of PDT monotherapy [30]. Wataru et al. reported that BCVA improved and was maintained through 5 years after the initial treatment of a combination of PDT and anti-VEGF injection [31]. Miyata et al. reported that BCVA significantly improved at 1 year and no difference between baseline BCVA and 5-year BCVA after both the initial treatment of a combination of PDT and anti-VEGF injection and anti-VEGF monotherapy [13]. In the present study, BCVA was maintained for 5 years. Some differences in the results between those studies may also be attributable to differences in the regimens of additional treatment. Furthermore, similarly to previous reports, MA influenced the visual outcomes in the present study [13, 16, 26].
Contrary to our expectation, the baseline RPE thickness in the MA group was not significantly lower than that in the non-MA group (P = 0.21). Although the RPE is constituted as a single layer of cells, the subfoveal RPE in Bietti crystalline dystrophy, in which the RPE is primarily degenerated, was thinner than that in retinitis pigmentosa and healthy controls [32]. Therefore, it would be considered that RPE thickness is an index to reflect RPE damage. However, the baseline RPE thickness was not a predictor for RPE atrophy (MA) in PCV.
Our study had some limitations. First, the sample size was relatively small because of the strict criteria for long-term observation in this study; thus, the study may have been under-powered to detect smaller differences between groups. In particular, 29 (40%) of 72 eyes were excluded because of loss to follow-up within 5 years. This rate was similar with that (50.7%) in a previous multicenter prospective cohort study of 5-year treatment outcome in PCV [33]. Second, swept-source optical coherence tomography (SS-OCT), which has longer wavelength and achieves greater tissue penetration, may be more accurate to measure the choroidal thickness now. However, SS-OCT was not commercially available in 2008; therefore, we used SD-OCT. Third, additional PRN treatment were judged individually by retinal specialists for real-world patients; thus, the number of anti-VEGF injections did not perfectly reflect the number of exudation events.
In conclusion, our long-term observation suggests that a thin subfoveal choroid at baseline and many followed intravitreal anti-VEGF injections in a PRN regimen increase the risk for development of MA involving the fovea 5 years after PDT.
Summary
What was known before
-
Five-year visual acuity was associated with MA in PCV. A risk factor of 2-year MA after anti-VEGF injection was baseline thin choroid in PCV.
What this study adds
-
Newly developed MA involving the fovea was observed in 41% of the eyes with PCV 5 years after the initial PDT. Risk factors of 5-year MA after PDT were thin choroid and number of additional anti-VEGF injections in PCV.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990;10:1–8.
Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002;86:321–7.
Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–15.
Hata M, Tsujikawa A, Miyake M, Yamashiro K, Ooto S, Oishi A, et al. Two-year visual outcome of ranibizumab in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefe’s Arch Clin Exp Ophthalmol. 2015;253:221–7.
Kim JH, Kim JW, Kim CG, Lee DW. Long-term switching between ranibizumab and aflibercept in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:1677–85.
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I, et al. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology 2015;122:1866–72.
Chan W-M, Lam DSC, Lai TYY, Liu DTL, Li KKW, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004;111:1576–84.
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2008;115:141–6.
Lai TYY, Lee GKY, Luk FOJ, Lam DSC. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 2011;31:1581–8.
Takayama K, Kaneko H, Kataoka K, Hattori K, Ra E, Tsunekawa T, et al. Comparison between 1-year outcomes of aflibercept with and without photodynamic therapy for polypoidal choroidal vasculopathy: Retrospective observation study. PLoS ONE. 2017;12:e0176100.
Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GOH. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120:835–44.
Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Investig Ophthalmol Vis Sci. 2002;43:830–41.
Miyata M, Ooto S, Yamashiro K, Tamura H, Hata M, Ueda-Arakawa N, et al. Five-year visual outcomes after anti-VEGF therapy with or without photodynamic therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2019;103:617–22.
Cho HJ, Kim K, Lim SH, Kang DH, Kim JW. Retinal pigment epithelial atrophy after anti-vascular endothelial growth factor therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2020;104:1443–7.
Abdelfattah NS, Zhang H, Boyer DS, Sadda SR. Progression of macular atrophy in patients with neovascular age-related macular degeneration undergoing antivascular endothelial growth factor therapy. Retina 2016;36:1843–50.
Kuroda Y, Yamashiro K, Ooto S, Tamura H, Oishi A, Nakanishi H, et al. Macular atrophy and macular morphology in aflibercept-treated neovascular age-related macular degeneration. Retina 2018;38:1743–50.
Kuroda Y, Yamashiro K, Tsujikawa A, Ooto S, Tamura H, Oishi A, et al. Retinal pigment epithelial atrophy in neovascular age-related macular degeneration after ranibizumab treatment. Am J Ophthalmol. 2016;161:94–103.
Hata M, Yamashiro K, Oishi A, Ooto S, Tamura H, Miyata M, et al. Retinal pigment epithelial atrophy after anti–vascular endothelial growth factor injections for retinal angiomatous proliferation. Retina 2017;37:2069–77.
Hikichi T, Kitamei H, Shioya S. Retinal pigment epithelial atrophy over polypoidal choroidal vasculopathy lesions during ranibizumab monotherapy. BMC Ophthalmol. 2016;16:55 https://doi.org/10.1186/s12886-016-0237-x
Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011;118:840–5.
Kim S-W, Oh J, Kwon S-S, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina 2011;31:1904–11.
Ford KM, Saint-Geniez M, Walshe T, Zahr A, D’Amore PA. Expression and role of VEGF in the adult retinal pigment epithelium. Investig Ophthalmol Vis Sci. 2011;52:9478–87.
Sadda SR, Tuomi LL, Ding B, Fung AE, Hopkins JJ. Macular atrophy in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology 2018;125:878–86.
Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN). Health Technol Assess. 2015;19:1.
Sadda SR, Guymer R, Monés JM, Tufail A, Jaffe GJ. Anti–vascular endothelial growth factor use and atrophy in neovascular age-related macular degeneration: Systematic Literature Review and Expert Opinion. Ophthalmology 2020;127:648–59.
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study∗. Am J Ophthalmol. 2015;159:915–24.
Schlötzer-Schrehardt U, Viestenz A, Naumann GOH, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe’s Arch Clin Exp Ophthalmol. 2002;240:748–57.
Nassisi M, Lavia C, Alovisi C, Musso L, Eandi CM. Short-term choriocapillaris changes in patients with central serous chorioretinopathy after half-dose photodynamic therapy. Int J Mol Sci. 2017;18:2468 https://doi.org/10.3390/ijms18112468
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317.
Miyamoto N, Mandai M, Oishi A, Nakai S, Honda S, Hirashima T, et al. Long-term results of photodynamic therapy or ranibizumab for polypoidal choroidal vasculopathy in LAPTOP study. Br J Ophthalmol. 2019;103:844–8.
Wataru K, Sugiyama A, Yoneyama S, Matsubara M, Fukuda Y, Parikh R, et al. Five-year outcomes of photodynamic therapy combined with intravitreal injection of ranibizumab or aflibercept for polypoidal choroidal vasculopathy. PLoS ONE. 2020;15:e0229231 https://doi.org/10.1371/journal.pone.0229231
Miyata M, Hata M, Ooto S, Ogino K, Gotoh N, Morooka S, et al. Choroidal and retinal atrophy of Bietti crystalline dystrophy patients with CYP4V2 mutations compared to retinitis pigmentosa patients with EYS mutations. Retina 2017;37:1193–202.
Akagi-Kurashige Y, Tsujikawa A, Yuzawa M, Ishibashi T, Nakanishi H, Nakatani E, et al. A 5-year multicenter prospective cohort study on the long-term visual prognosis and predictive factors for visual outcome in Japanese patients with age-related macular degeneration: the AMD2000 study. Jpn J Ophthalmol. 2018;62:137–43.
Funding
This work was supported in part by a grant-in-aid for scientific research (no. 18K09444 and no. 21K09716) from the Japan Society for the Promotion of Science, Tokyo, Japan. This organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
KK is responsible for the study design and writing the paper, MM is responsible for study design, data acquisition, and writing the paper. SO, HT, NUA, AT, AU, YM, MM, KY and AT are responsible for data acquisition and review of the paper.
Corresponding author
Ethics declarations
Competing interests
All authors attest that they meet the current ICMJE criteria for authorship.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kawai, K., Miyata, M., Ooto, S. et al. Macular atrophy at 5 years after photodynamic therapy for polypoidal choroidal vasculopathy. Eye 37, 1067–1072 (2023). https://doi.org/10.1038/s41433-022-02067-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02067-6
This article is cited by
-
Intravitreal anti-vascular endothelial growth factor and combined photodynamic therapy for pachychoroid neovasculopathy: long-term treatment outcomes
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Two-year clinical outcomes of triple therapy with photodynamic therapy, anti-vascular endothelial growth factor agent, and triamcinolone acetonide for neovascular age-related macular degeneration
Japanese Journal of Ophthalmology (2023)