Abstract
Objectives
To analyse the longitudinal changes in visual acuity and risk factors for recurrence or development of choroidal neovascularisation (CNV) in eyes with acute or chronic central serous chorioretinopathy (CSCR).
Methods
This was a retrospective, multicentric, longitudinal, observational study done in patients with a diagnosis of unilateral or bilateral CSCR and having at least 4 years of follow-up between the years 1999 and 2020. Kaplan–Meier curves were used for assessing cumulative risks. Multivariate logistic, linear and cox regression models were used for risk factor analyses. The trend in visual acuity, cumulative risks of recurrence and CNV formation was analysed.
Results
A total of 117 out of 175 eyes (66.8%) had stable or improvement in vision at last follow-up, while 24 eyes had more than/equal to 3 line loss of vision. Four eyes (7.7%) with acute CSCR at initial presentation developed features of chronic CSCR at the final presentation. Thirty-seven eyes had recurrence during the follow-up with a 10-year cumulative recurrence rate of around 30%. On Cox proportional hazard regression analysis, history of previous treatment and male gender (p = 0.03) were associated with a lower risk of recurrence. Twenty-four developed de novo CNV by the end of follow-up and higher age (p = 0.001) and a higher number of recurrences (p = 0.05) were associated with a higher risk of early de novo CNV formation. The cumulative 10-year CNV development rate was 17.4%.
Conclusion
A non-temporal relationship between acute and chronic CSCR was seen. Previous treatment, smoking and baseline RPE abnormality affected recurrence of SRF or CNV formation.
Similar content being viewed by others
Introduction
First described by von Graefe in 1866, central serous chorioretinopathy (CSCR) is defined as idiopathic serous macular detachment due to the collection of sub-retinal fluid (SRF) [1]. It is believed to be a disease primarily of the choroid that has been demonstrated with the help of several angiographic and optical coherence tomography (OCT)-based studies [2,3,4]. Since its first description, a multitude of retrospective and prospective studies have emerged describing the clinical characteristics and management. Patients often present with variable degrees of visual disturbance and metamorphopsia [5], and although CSCR is a self-limiting disease with a good visual prognosis [6, 7], there is a significant proportion of eyes that demonstrate multiple recurrences and many more that manifest a persistent fluid despite treatment [8, 9]. These recurrences and persistence often lead to irreversible vision loss or morbidity over time. Thus, it is imperative to analyse eyes with long-term follow-up in order to better understand the basis of such deviation from the normal course.
Most of the studies describing the long-term follow-ups and outcomes either date back to the era before OCT or employ a very small sample size [8, 10]. These works used fluorescein angiography and focused on the visual outcome and recurrence rates. However, there are several recent short-term studies that have described changes in the outer retina, RPE and choroid in CSCR with the help of multimodal imaging [11, 12]. These changes can influence the visual and structural outcome of the disease. In addition to this, treatment of CSCR varies significantly among ophthalmologists. While the resolution of SRF and improvement in vision has been seen with these treatments, long-term outcomes can be variable and have been rarely compared. This study aims at analysing the longitudinal changes in visual acuity and imaging characteristics in CSCR eyes in an attempt to understand the risk factors that determine the final visual outcome.
Methods
This was a retrospective, multicentric, longitudinal, observational study done in eyes with unilateral or bilateral CSCR. It adhered to the tenets of the Declaration of Helsinki and was approved by the local institutional review boards or ethics committee. Written consent was taken from all patients before enrolling in the study. The inclusion criteria were patients above the age of 18 with no history of ocular surgery or systemic co-morbidity, having a diagnosis of unilateral or bilateral acute or chronic CSCR with at least 4 years follow-up. We excluded patients with high myopia, optic disc pathology and retinal degenerations/dystrophies or vasculopathies that could affect the imaging characteristics. Eyes that did not have sufficient documentation of OCT and FFA images were excluded. A total of 175 eyes 146 patients (106 males and 40 females) were included. Several demographic parameters such as age, gender, history of steroid use, smoking, sleep alteration, type of CSCR, previous treatment and duration of symptoms; and imaging parameters like presence of intra-retinal fluid (IRF) and SRF, central macular thickness, choroidal thickness, presence of pachyvessel, double-layer sign (DLS), and RPE alterations were analysed. Acute CSCR was defined as SRF persisting for less than 4 months and chronic CSCR was defined for eyes with more than 4 months of disease [13]. Choroidal thickness was manually measured by using an in-built calliper tool by drawing a perpendicular vector from the outer edge of the hyper-reflective RPE to the inner sclera (choroid–sclera junction) within 500 microns of the fovea. DLS was defined as irregular shallow PEDs with hypo or hyper-reflective content inner to an intact hyper-reflective Bruch membrane seen on any horizontal line scan passing through the fovea and was represented in microns. Recurrence was defined as any episode of reappearance of SRF on OCT in a particular year, after the complete resolution, with/without a decrease in vision. A visit with the best vision in a year was included as a representative visit for that particular year. In case of recurrence or CNV detection, the first visit with recurrence/CNV was taken as the representative visit for the year.
Visual acuity
Best-corrected visual acuity (BCVA) measurements at the baseline and then every year thereafter were retrieved. For the yearly measurements, the best visual acuity documented in the year was taken. Visual acuity trends in eyes that had undergone treatment and managed with observation were charted separately. Visual outcomes of each treatment modality were also calculated and compared. Several visual acuity parameters were also assessed for any possible baseline predictors, using linear and binary logistic regression analysis.
Recurrence and CNV formation
Binary logistic regression was performed to look for factors affecting recurrence and CNV development. Rates of recurrence and development of CNV were also charted using Kaplan–Meier (KM) curves. KM curves for recurrence were prepared against different treatment modalities separately and compared. Cox proportional hazard regression analysis was performed for recurrence and de novo CNV formation. Binary logistic regression was also carried out to look for baseline factors affecting the presence of CNV (pre-existing plus de novo CNV).
Statistical analysis
Statistical analysis was done SPSS statistical software version 20 (SPSS, Inc., Chicago, IL, USA) and graphs were prepared using GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA). Continuous variables were reported as mean ± standard deviation. Comparison of baseline and final values were done using Wilcoxon signed rank test. A p value of <0.05 was taken as statistically significant.
Results
The mean age of the 146 patients was 52.9 ± 12.5 years (majority of the patients were Caucasians). Fifty-two eyes were acute and 123 had chronic CSCR at presentation. Twenty-nine patients had a bilateral presentation. The mean duration of symptoms was 17.9 ± 31.1 months. A total of 94 eyes were managed with observation only, while 16 were offered focal laser, 5 were offered micropulse, 18 were managed with PDT, 18 were given oral eplerenone, 16 patients received anti-VEGF injections at baseline and eight eyes received combination therapy. Treatment of CSCR was at the discretion of the treating ophthalmologist and was based on angiography findings. Eyes with CNV were either managed with PDT or anti-VEGF. Four patients (7.7%) out of the 52 eyes with acute CSCR had features of chronic CSCR at the final presentation after a mean follow-up of 6.3 ± 3.7 years.
Visual acuity
The mean baseline BCVA was 0.29 ± 0.36 logMAR (Snellen equivalent 20/40) (0.16 ± 0.19 logMAR for acute CSCR and 0.34 ± 0.40 logMAR for chronic CSCR) and the final BCVA at the end of a mean of 6.6 ± 3.7 years follow-up was 0.29 ± 0.41 logMAR (Snellen equivalent 20/40) (p = 0.76) (0.16 ± 0.22 logMAR for acute CSCR and 0.35 ± 0.46 logMAR for chronic CSCR). While 117 eyes had an improvement in or stable vision at last follow-up, 24 eyes (4 acute and 20 chronic CSCR eyes) had more than/equal to 3 line loss of vision. The baseline visual acuity of eyes that had a decrease in vision at last follow-up was 0.21 ± 0.27 logMAR, while that of eyes that gained vision at the last follow-up, was 0.33 ± 0.41 logMAR (the difference was statistically significant, p = 0.02) (Fig. 1A). The subset of eyes that lost more than/equal to 3 lines, had a baseline BCVA of 0.27 ± 0.32 logMAR. On further analysis of this sub-group, 11 eyes had CNV, 3 eyes had multiple recurrences, 2 eyes had chronic persistence of fluid (2 eyes) and 8 eyes had extensive RPE/outer retinal atrophy involving fovea. Eyes that underwent treatment in the first year had greater improvement in visual acuity (mean decrease in 0.26 logMAR) than those that were observed (mean increase in 0.11 logMAR) (Fig. 1B). A breakdown of baseline characteristics of different treatment modalities has been provided in Table 1.
Recurrence of CSCR
A total of 37 eyes had at least one recurrence during the follow-up (eight eyes had two or more recurrences). The median time to recurrence was 3 years (IQR 2–5 years). In eyes with a diagnosis of acute CSCR at baseline, a total of 9 eyes (17.3%) had a recurrence, while in eyes with chronic CSCR, 29 eyes (23.6%) had a recurrence. KM survival predicted that the 10-year cumulative recurrence rate was around 30% (28.8%) (Fig. 2). KM curve for recurrence was also plotted for the group who received treatment at baseline versus those who were observed. Although the 10-year cumulative recurrence rate for the treatment arm (22.5%) was lower than that of the observation arm (32.4%), it was not found to be statistically significant (log-rank test p value = 0.39) (Fig. 2).
The recurrence rate in observation group was 26.6% (25/94), 16.6% (3/18) in medication group, 33.3% (7/21) in laser group, 0.5% (1/18) in PDT group, 12.5% (2/16) in injection group and none in combination group. The difference in survival curves among the treatment modalities was not statistically significant. On Cox proportional hazard regression analysis, factors associated with a higher survival time were history of previous treatment (p = 0.05) and male gender (p = 0.03) on univariate analysis. Male gender (p = 0.03) was the only factor found to be significant in multivariate analysis.
CNV formation
The mean age of patients in the CNV group was 61.5 ± 10.8 years (range 38–81 years). Choroidal thickness in these eyes at baseline was 343.8 ± 76.5 microns. Nine eyes had a CNV at baseline, out of which eight eyes had stabilised/improved at the end of follow-up. Twenty-four developed de novo choroidal neovascularisation by the end of the last follow-up and it included 22 chronic CSCR eyes and two acute CSCR case. Factors affecting the presence of CNV were chronic CSCR (p = 0.01), higher age (p < 0.001), smoking (p < 0.001), sleep/psychological disturbance (p = 0.02), presence of IRF (0.02) and presence of DLS (p = 0.05). However, on multivariate analysis, only age (p = 0.01) and smoking (p = 0.01) were found to be significant.
On KM analysis, the cumulative 10-year CNV development rate was 17.4%. It predicted that around 75% of the chronic CSCR cases will not develop a CNV at the end of 20 years. On Cox proportional hazard regression analysis, factors associated with a higher risk of early de novo CNV formation were increased age (p = 0.001) and a higher number of recurrences (p = 0.05). The presence of DLS was almost significant (p = 0.06) in multivariate analysis.
Imaging parameters
The mean baseline CMT was 375.7 ± 148 microns and the baseline CT was 354.4 ± 74.8 microns. Mean CMT and CT values at the final visit were 268.3 ± 176.8 microns and 343.2 ± 109.4 microns, respectively. A total of 46 eyes (26.3%) had a DLS at baseline, out of which only 9 had a CNV network at baseline. Focal RPE alteration was present in 73 eyes, with the remaining 102 eyes having multifocal alterations. IRF was present in 15 eyes at baseline and a pachyvessel on OCT could be visualised in 44 eyes. On FFA, multiple leaks were seen in as many as 123 eyes. Several baseline factors were evaluated against visual acuity outcomes, recurrence and CNV formation, using binary, linear and cox regression models and a summary of the results have been shown in Table 2. Other than baseline vision, none of the baseline parameters had any predictive role in the absolute values of final visual acuity (p < 0.001).
Discussion
The study highlighted the structural and functional changes that occur over time in eyes with CSCR. Among eyes with acute CSCR, only 7.7% went on to develop chronic CSCR feature after a mean follow-up of 6.3 ± 3.7 years. We also found a 10-year cumulative recurrence rate of around 30% and a 10-year cumulative CNV formation rate of around 20%. A higher age, higher duration of follow-up and observation had a higher association of vision loss; history of previous treatment was associated with lower recurrence; and higher age and higher number of recurrences were associated with a higher risk of CNV formation.
Acute CSCR has been defined as a neurosensory detachment that resolves within 4 months of the development of symptoms [13]. On the other hand, the term chronic CSCR has been traditionally applied to describe eyes with SRF persisting for more than 4 months [13]. Almost 30% of the eyes in our cohort had acute CSCR at presentation. We found that 12 eyes (23.1%) in this group had long-standing SRF on follow-up. However, Daruich et al. suggested that it is better to refer to these entities as ‘non-resolving’ or ‘persistent’ and reserve the term ‘chronic CSCR’ for a more widespread RPE abnormality with variable outer retinal damage [13]. Considering this definition, only 4 (7.7%) of the eyes with acute CSCR at presentation, had features of chronic CSCR at the final presentation. These findings suggest a non-temporal relationship between acute and chronic CSCR and that they might be two different entities altogether. In a recently proposed classification by the Central Serous Chorioretinopathy international group, CSCR was divided into simple and complex based on the degree of RPE involvement in order to have a better distinction between the morphological variants [14]. Sub-types such as bullous CSCR and RPE tears were sub-grouped into atypical CSCR due to their unpredictable course and poor prognosis. Although it addressed a lot of controversies related to the traditional classification, it is yet to be determined whether this will make it easier to monitor or predict the progression of this enigmatic disease.
CSCR typically has a good visual prognosis with the recovery of most visual disturbances after resolution of SRF [6, 7]. However, a significant subset of eyes develops gross reduction in central visual acuity even after complete resolution of SRF [15]. Thus, SRF, although playing a pivotal role, is not the sole factor responsible for the varied prognosis. Several factors such as age, systemic risk factors, personal habits, etc., have been described to modulate the severity of the disease [16]. The patients in our cohort overall had a good mean visual acuity at the end of the follow-up (initial and final mean BCVA was 20/40 Snellen). However, on sub-classifying the eyes based on whether treatment was given or not, it was seen that eyes that were observed in the first year, had a poorer visual acuity at the end of follow-up than eyes that were treated. A stable or improvement in vision was achieved in a significant proportion of eyes (66.9%). Out of the 58 eyes that had a decrease in vision, 24 eyes (41.4%) had more than/equal to 3 line loss of vision. Even though this subset had a good mean baseline visual acuity, the loss in vision in these eyes was due to CNV, recurrence, persistence of fluid or RPE/outer retinal atrophy involving fovea. Although there is only a small chance of this, the development of moderate or severe vision loss is not uncommon in the disease. Thus, evaluating baseline prognosticating factors is an important strategy during follow-up. Several visual acuity parameters were analysed against the baseline factors. In our study, an increased age, higher duration of follow-up and observation (compared to laser only) had a higher association with a drop in vision while a better baseline visual acuity and persistence of fluid were associated with lesser gain in visual acuity. Again, factors associated with a mod-severe vision loss were persistence of fluid, CNV formation and higher age at baseline.
Long-standing SRF, be it due to recurrence or persistence of fluid, can cause irreversible damage to the photoreceptors [9] and in spite of multiple therapeutic options, a significant proportion of eyes suffer from multiple recurrences. Thus, identifying risk factors of recurrence can aid in treatment planning and counselling. Yu et al. suggested that male gender, older age and poor sleep behaviour are associated with higher recurrence and thus require earlier treatment [17]. History of previous treatment was found to be an important factor associated with higher survival time (lower risk) on univariate cox regression analysis. Therefore, treatment appears to have a definite advantage over observation with respect to time to relapse. This is also reflected to some extent in the KM graph for recurrence. Figure 2 demonstrates a 10-year cumulative recurrence rate of around 30% overall and a slight advantage of treatment over observation (although the difference was not statistically significant on log-rank test). The lowest rate of recurrence was seen in the combination and PDT groups, whereas a high recurrence was seen in the observation and laser group (although the difference in the KM curve was not significant). Considering the high percentage of chronic CSCR in our cohort, conventional laser appears to be less effective in tackling the diffuse choroidal leakage and RPE abnormalities. This was quite different from that described by a previous study by Lai et al., where the authors reported a rate of 53% in untreated CSCR eyes and 20% in eyes treated with PDT [18]. One possible explanation could be the different baseline characteristics in both studies. While almost 30% of eyes in our study had acute CSCR, this differentiation was lacking in the study by Lai et al. PDT has already been established as one of the most effective treatment modalities in CSCR [19, 20]. By directly targeting the hyperpermeable choroidal vessels, PDT causes vascular remodelling, choroidal hypoperfusion and narrowing of choriocapillaris [21]. This mechanism gives PDT a clear advantage over other treatment options in terms of recurrence, either as a monotherapy or in combination, as demonstrated from the results of our study. A persistent CSCR can be as hazardous as a recurrence. factors associated with a higher risk of persistence of fluid were smoking and higher baseline RPE abnormalities. Smoking as a risk factor of CSCR is well established [22]. By-products released into the systemic circulation can lead to local vascular and metabolic alterations that can lead to a multitude of ocular and systemic diseases. Although the exact pathogenesis is still debatable, chronic nicotine use has been described to cause alterations in nitric oxide signalling of Ca2+ channels in vascular structure [23], which could predispose to non-response/persistence of fluid. Similarly, widespread RPE alterations could potentially impede SRF reabsorption, thereby leading to a persistence of the disease.
CNV, although rare in CSCR, was also found to be associated with the persistence of fluid. The incidence of CNV in chronic CSCR has been described to be around 9–45%, detected by multimodal imaging [24,25,26,27]. While its presence is often obscured by background RPE hyperfluorescence on FFA, OCTA provides excellent delineation of the vascular network. Its detection is of significant value considering the fact that response to treatment is usually variable [28,29,30]. Some of the most important associations of detection of CNV in our study were found to be chronic CSCR, higher age, smoking, sleep/psychological disturbance, presence of IRF and presence of DLS (only age and smoking were significant in multivariate analysis). Patients with CNV had a slightly higher age at baseline than those that did not have CNV. While there was an overlap of this age range with those that we see in age-related macular degeneration (AMD), exclusion of AMD related CNV was based on various multimodal imaging findings such as the presence of pachychoroid (with or without pachyvessel), RPE alterations, that we commonly seen in CSCR. One of the important characteristics was the presence of IRF. Also described as posterior cystoid macular degeneration, the presence of these cysts has usually been described to respond poorly to treatment and be associated with a poor visual outcome [31, 32]. Iida et al. suggested that it forms secondary to chronic detachment of neurosensory retina and ischaemic insult to the outer retina [32]. A recent publication on OCTA-based analysis also demonstrated a higher prevalence of CNV network in these eyes than that described in other forms of CSCR [26]. Whether these cysts are a result of direct exudation into the intra-retinal compartment from the CNV network, or just a coincidental finding in eyes with outer retinal ischaemia, is still unknown. The presence of CNV was also associated with the persistence of SRF in our study, which if missed may cause undue delay in adequate treatment. Thus, care should be taken to look for these networks in cases of non-responding or persistent SRF. Nine eyes (6.9%) in our cohort had a choroidal neovascular membrane at baseline, while 24 (13.7%) developed de novo CNV during follow-up. KM curve demonstrated a 10-year cumulative rate of around 25%. The model also predicted that as many as 70% of eyes would not develop a CNV at the end of 20 years. This could suggest that most of the cases of CNV that appear de novo do so in the first ten years of presentation. This information could aid in monitoring and treatment planning. However, we understand that advanced imaging techniques have contributed to the early detection of CNV, particularly in association with CSCR.
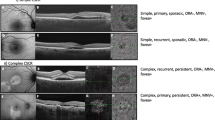
One of the most commonly encountered OCT findings at the level of RPE is the DLS. Some authors suggest that these DLS may harbour a CNV network and thus should undergo a thorough clinical evaluation [33, 34]. While 46 eyes (26.3%) had a DLS in our study, only 9 of them had a CNV network at baseline. The content of DLS may be hyper-reflective or hypo-reflective. Although the most common form of DLS seen in CSCR is a uniformly hypo-reflective DLS, a hyper-reflective DLS could suggest an underlying CNV network like that seen in PNV and PCV (Fig. 3) [33, 35]. However, it is still unknown whether the hypo-reflective DLS represents an early form of CNV or the both develop independently. A significant association between the presence of DLS at baseline and development of de novo CNV (univariate analysis) on follow-up in our study gave us an indication of a possible temporality of these two variations.
A Optical coherence tomography (OCT) of an eye with a double-layer sign with hyper-reflective content (arrow) and OCT angiography of the same eye showing a vascular network (asterisk) on the outer retinal slab. B Example of an eye with hypo-reflective DLS (arrowhead) on OCT and no detectable network on OCT angiography.
The current study had its own share of limitations of being a retrospective study that resulted in difficulty in assessing temporal associations in a lot of variables. Second, outcome analysis based on ethnicity was not performed. As a majority of patients in the study were Caucasians, a sub-group analysis based on ethnicity would not have made any impact on the overall outcome. Third, due to the multicentric nature of the study, choice of imaging and management differed between the centres, which could have resulted in misclassification bias. Also, the sub-division of cases at baseline into acute and chronic CSCR was based on the self-reported duration of symptoms, and thus could have resulted in inaccuracies in baseline characteristics. Lastly, evolving imaging techniques in the last decade must have also influenced the various parameters, thus influencing the treatment strategies and outcome. Nonetheless, this study is one of the largest, multicentric data, utilising both functional and imaging-based characteristics to monitor changes in eyes with CSCR during long-term follow-up.
In conclusion, the study reports a non-temporal relationship between acute and chronic CSCR with several factors such as history of previous treatment, smoking and baseline RPE abnormality playing an important role in recurrence or persistence of SRF. Of note, was the association of CNV development with increased age, smoking and number of recurrences. We also advocate to carefully look for a CNV network in cases of persistent fluid, IRF or a DLS. Considering the small percentage of eyes in acute CSCR that developed significant RPE alterations and shift to chronicity, regular and long follow-up should be considered in acute CSCR even after resolution. Although this study tried to cater to a lot of unanswered questions on the clinical course and outcomes, larger, prospective, image-based studies would be required in the future to decipher the true nature of this enigmatic disease.
Summary
What was known before
-
Central serous chorioretinopathy can present in acute or chronic forms. Although usually self-limiting, the course of the disease can get complicated by multiple recurrences and CNVM formation.
What this study adds
-
A non-temporal relationship between acute and chronic CSCR was seen. History of previous treatment, smoking, and baseline RPE abnormality play an important role in the recurrence of SRF or CNVM formation.
Data availability
The datasets generated during and/or analysed during the study are available from the corresponding author on reasonable request.
References
Von Graefe A. Ueber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:211–5.
Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429.
Yang L, Jonas JB, Wei W. Optical coherence tomography–assisted enhanced depth imaging of central serous chorioretinopathy. Inv Ophthalmol Vis Sci. 2013;54:4659–65.
Jirarattanasopa P, Ooto S, Tsujikawa A, Yamashiro K, Hangai M, Hirata M, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119:1666–78.
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Act Ophthalmol. 2008;86:126–45.
Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22:166–73.
Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91:247–50.
Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–20.
Sartini F, Figus M, Nardi M, Casini G, Posarelli C. Non-resolving, recurrent and chronic central serous chorioretinopathy: available treatment options. Eye. 2019;33:1035–43.
Kanyange M, De Laey J. Long-term follow-up of central serous chorioretinopathy (CSCR). Bull Soc Belg Ophtalmol. 2002;284:39–44.
Ferrara D, Mohler KJ, Waheed N, Adhi M, Liu JJ, Grulkowski I, et al. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology. 2014;121:719–26.
Zola M, Chatziralli I, Menon D, Schwartz R, Hykin P, Sivaprasad S. Evolution of fundus autofluorescence patterns over time in patients with chronic central serous chorioretinopathy. Act Ophthalmol. 2018;96:e835–e9.
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118.
Chhablani J, Cohen FB, Aymard P, Beydoun T, Bousquet E, Daruich-Matet A, et al. Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retin. 2020;4:1043–6.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–88.
Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case-Control Study Group. Risk factors for central serous chorioretinopathy: a case–control study. Ophthalmology. 2004;111:244–9.
Yu J, Xu G, Chang Q, Ye X, Li L, Jiang C, et al. Risk factors for persistent or recurrent central serous chorioretinopathy. J Ophthalmol. 2019;2019:5970659.
Lai TYY, Wong RLM, Chan W-M. Long-term outcome of half-dose verteporfin photodynamic therapy for the treatment of central serous chorioretinopathy (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T8.
Doyle J, Gupta B, Tahir I. Long term outcomes for patients treated with half-fluence photodynamic therapy for chronic central serous chorioretinopathy: a case series. Int J Ophthalmol. 2018;11:333.
Lai FH, Ng DS, Bakthavatsalam M, Chan VC, Young AL, Luk FO, et al. A multicenter study on the long-term outcomes of half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2016;170:91–9.
Chan W, Lam D, Lai T, Tam B, Liu D, Chan C. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–8.
Türkcü FM, Yüksel H, Şahin A, Cinar Y, Cingü K, Arı Ş, et al. Effects of smoking on visual acuity of central serous chorioretinopathy patients. Cutan Ocul Toxicol. 2014;33:115–9.
Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca2+ channels in cerebral arterioles. Circ Res. 2001;88:359–65.
Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149:361–3. e1
Stattin M, Ahmed D, Forster J, Glittenberg C, Herrmann M, Krebs I, et al. Detection of secondary choroidal neovascularization in chronic central serous chorioretinopathy by swept source‐optical coherence tomography angiography. Act Ophthalmol. 2019;97:e135–e6.
Sahoo NK, Mishra SB, Iovino C, Singh SR, Munk MR, Berger L, et al. Optical coherence tomography angiography findings in cystoid macular degeneration associated with central serous chorioretinopathy. Br J Ophthalmol. 2019;103:1615–8.
Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–80.
Ergun E, Tittl M, Stur M. Photodynamic therapy with verteporfin in subfoveal choroidal neovascularizationsecondary to central serous chorioretinopathy. Arch Ophthalmol. 2004;122:37–41.
Chhablani J, Kozak I, Pichi F, Chenworth M, Berrocal MH, Bedi R, et al. Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina. 2015;35:2489–97.
Sacconi R, Tomasso L, Corbelli E, Carnevali A, Querques L, Casati S, et al. Early response to the treatment of choroidal neovascularization complicating central serous chorioretinopathy: a OCT-angiography study. Eye. 2019;33:1809–17.
Piccolino FC, De La Longrais RR, Manea M, Cicinelli S, Ravera G. Risk factors for posterior cystoid retinal degeneration in central serous chorioretinopathy. Retina. 2008;28:1146–50.
Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23:1–7.
Sheth J, Anantharaman G, Chandra S, Sivaprasad S. “Double-layer sign” on spectral domain optical coherence tomography in pachychoroid spectrum disease. Ind J Ophthalmol. 2018;66:1796.
Hagag AM, Rasheed R, Sivaprasad S. The significance of double layer sign in eyes with choroidal neovascularization secondary to central serous chorioretinopathy. Inv Ophthalmol Vis Sci. 2021;62:1922.
Chhablani J, Mandadi SK. Commentary: “double-layer sign” on spectral domain optical coherence tomography in pachychoroid spectrum disease. Ind J Ophthalmol. 2019;67:171.
Author information
Authors and Affiliations
Contributions
JC: design; NKS, JO, AS, DM, NGR, SM: conduct of the study; JO, AS, DM, NGR, SM: collection; RS, RV, BT, LHL, VP, GA, AMC, GL-G, NW, EB, GQ: management; NKS, JO, AS, DM, NGR: analysis; NKS, JO: interpretation of the data; NKS, JO: preparation; RS, RV, BT, LHL, VP, GA, AMC, GL-G, NW, EB, GQ, JC: review; RS, RV, BT, LHL, VP, GA, AMC, GL-G, NW, EB, GQ, JC: approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahoo, N.K., Ong, J., Selvam, A. et al. Longitudinal follow-up and outcome analysis in central serous chorioretinopathy. Eye 37, 732–738 (2023). https://doi.org/10.1038/s41433-022-02044-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02044-z
This article is cited by
-
Pathomechanisms in central serous chorioretinopathy: A recent update
International Journal of Retina and Vitreous (2023)
-
Ten-year follow-up and sequential evaluation of multifocal retinal pigment epithelium abnormalities in central serous chorioretinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)