Abstract
Objectives
To investigate whether SARS-CoV-2 causes morphological changes in the corneal sub-basal nerve plexus (CSNP) of post-COVID-19 patients using in vivo confocal microscopy (IVCM).
Methods
A total of 70 participants were included in the study and were divided into three groups. Post-COVID-19 patients with neurological manifestations were considered Group 1 (n = 24), and post-COVID-19 patients without neurological manifestations were considered Group 2 (n = 24). Healthy control participants were considered Group 3 (n = 22). The parameters of the CSNP, including nerve fibre density (NFD), nerve branch density (NBD), and nerve fibre length (NFL), were investigated in all participants using IVCM. Additionally, corneal sensitivity was tested by corneal esthesiometry.
Results
The mean NFD, NBD, and NFL values of Group 1 (16.12 ± 4.84 fibre/mm2, 27.97 ± 9.62 branch/mm2, and 11.60 ± 2.89 mm/mm2) were significantly lower than those of Group 2 (19.55 ± 3.01 fibre/mm2, 40.44 ± 7.16 branch/mm2, and 15.92 ± 2.08 mm/mm2) and Group 3 (25.24 ± 3.75 fibre/mm2, 44.61 ± 11.80 branch/mm2, and 17.76 ± 3.32 mm/mm2) (p < 0.05 for all). Except the mean NFD value (p < 0.001), there were no significant differences in terms of the mean NBD and NFL values between Group 2 and Group 3 (p = 0.445, p = 0.085). The value of the mean corneal sensitivity was significantly higher in Group 3 (59.09 ± 1.97 mm) compared to Group 1 (55.21 ± 1.02 mm) and Group 2 (55.28 ± 1.18 mm) (p < 0.001, p < 0.001) but there was no significant difference between Group 1 and Group 2 (p = 1.000).
Conclusion
In post-COVID-19 patients, the mean parameters of CSNP were lower than in the control group. These differences were more pronounced in patients who had neurological manifestations of COVID-19.
Similar content being viewed by others
Introduction
A deadly and infectious new coronavirus was detected in Wuhan City, China, in December 2019 [1]. This novel infection, coronavirus disease 2019 (COVID-19), spread quickly to all countries over time and led to a pandemic. Researchers have rapidly begun to investigate this new infectious disease and it has been shown that COVID-19 pathogen, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cause damage to nerve tissues [2].
It has been announced that the loss of smell and taste is probably the initial symptom of COVID-19 [3]. Also, cranial neuropathies, including trochlear, abducens, and facial nerve palsies, have been published in the literature [4,5,6,7]. It was also found that ocular manifestations, including conjunctivitis, epiphora, and chemosis, occurred in many patients with COVID-19 [8]. In some patients, it was reported that ocular complaints were the first signs of COVID-19 [9].
On the other hand, the cornea, a dense tissue in terms of nerve fibres innervated by the trigeminal nerve and its nerve fibres, can be affected by viral diseases. For instance, a significant decrease in nerve fibre density (NFD) of the corneal sub-basal nerve plexus (CSNP) was shown in eyes with herpes simplex virus keratitis and uveitis using in vivo confocal microscopy (IVCM) [10]. IVCM allows real-time imaging of living human corneas without causing tissue damage, and it is widely used in clinical practice to evaluate corneal and ocular surface pathologies [11]. Using the high axial and lateral resolution of IVCM, an in vivo examination of cellular changes in corneal pathologies is possible [11]. The NFD, nerve branch density (NBD), and nerve fibre length (NFL), which are defined as indicators of neuropathological changes in the cornea, can be quantitatively evaluated using this technique [12].
On this basis, the purpose of this study was to demonstrate, using IVCM, whether SARS-CoV-2 causes morphological changes in the CSNP of recovered COVID-19 patients (post-COVID-19 patients).
Materials and methods
Necessary permission was obtained from the Turkish Ministry of Health to carry out this cross-sectional comparative study. The Clinical Research Ethics Committee of the Meram School of Medicine, Necmettin Erbakan University approved this study, and adhered to the tenets of the Declaration of Helsinki (No: 2021/3131). Each participant signed written informed consent forms after they were provided with detailed information about the study.
Post-COVID-19 patients 18–50 years of age who applied to the ophthalmology department of the Meram School of Medicine, Necmettin Erbakan University between March 2021 and April 2021 were included in this study. The inclusion criteria were as follows: (1) having a history of a positive COVID-19 reverse transcriptase-polymerase chain reaction (RT-PCR) test from a nasopharyngeal swab previously, (2) having symptoms (e.g. fever, dyspnoea, dry cough, and fatigue, etc.) during the COVID-19, (3) having a negative COVID-19 RT-PCR test from a nasopharyngeal swab at least 1 month before inclusion in the study. Patients with the following conditions were not included in the study: (1) patients who are asymptomatic during the COVID-19; (2) patients with a history of ocular surgeries, ocular surface diseases, such as chemical or thermal injury; (3) patients with a history of ocular anterior segment pathology, including any conjunctival or corneal diseases; (4) patients taking any topical or systemic medication that may affect the tear film or meibomian gland function; (5) patients using contact lenses; (6) patients with ≥± 3 D spherical equivalent refractive error; (7) patients with any systemic diseases that may affect CSNP, including diabetes mellitus and neurodegenerative diseases; (8) patients with pregnancy or lactation; and (9) patients with a smoking or alcohol consumption history.
The neurological manifestations of COVID-19, including loss of taste and smell, ophthalmoparesis, peripheral neuropathy and myopathy, and cerebrovascular diseases, as reported in the study of Romero-Sánchez et al. were questioned. After receiving detailed anamnesis, post-COVID-19 patients were divided into two groups according to whether they had neurological manifestations during the COVID-19 [13]. Those patients with neurological manifestations were included in Group 1, and those without neurological manifestations were included in Group 2. Age- and sex-matched patients admitted to our ophthalmology department for routine ophthalmologic examination were included in Group 3 (control group). All participants underwent a comprehensive ophthalmologic examination, including best-corrected visual acuity, slit-lamp examination, intraocular pressure measurements with a noncontact tonometer, and fundus examination. Participants with detected or suspected ophthalmologic pathology were eliminated from the study. Based on these criteria, 24 patients were included in Group 1, 24 patients in Group 2, and 22 participants in Group 3.
The Cochet-Bonnet esthesiometer (Cochet-Bonnet; Luneau, France) was used to measure the corneal sensitivity in all participants. The measurements were performed by the same experienced researcher (RO). In this technic, a nylon monofilament was gently applied to the centre of the cornea. The starting length of the filament was 60 millimetres (mm) and it was gradually reduced in 5-mm steps until the first response is seen. The test was repeated 2 more times at the longest filament level with a positive response and the results were noted as the level of corneal sensitivity.
The corneas of all participants were evaluated with the Heidelberg Retina Tomograph III (HRT III) with the Rostock Cornea Module (RCM) (Heidelberg Engineering, Heidelberg, Germany) under topical anaesthesia (0.5% proparacaine HCl; Alcaine®; Alcon Laboratories, Fort Worth, TX, ABD). IVCM was performed under similar conditions by the same experienced researcher (AOG). The images of the CSNP could be viewed on the scanner screen during scanning, and these images were digitally recorded. Images from the central cornea with a size of 400 × 400 μm were obtained with this technique. The corneal images of one randomly selected eye of all participants were considered for the assessment. In total, 38 right eyes and 32 left eyes of 70 participants were included in the evaluation. Three to five good-quality images of CSNP were selected for each eye included in the study.
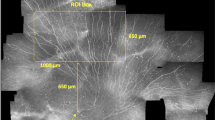
In this study, the NFD, NBD, and NFL parameters of CSNP were evaluated, as described previously in the study of Malik et al. [12]. Two independent researchers, blinded to the groupings and clinical information (EM and SB), evaluated all selected images. An open-source semi-automated plugin (Neuron J by Erik Meijering; ver. 1.4.3) for Image J software (Wayne Rasband and collaborators, National Institutes of Health, USA; ver. 1.53i) was used to trace the nerve fibres and branches (Fig. 1). After tracing the nerves, the summed length of the nerves calculated by the software was converted to mm/mm2 and noted as NFL. Nerve fibres and branches in the images were counted manually, calculated as fibres/mm2 and branches/mm2, and indicated as NFD and NBD, respectively. Average values of the parameters mentioned above were obtained for each eye by taking the average values calculated in the selected images of the same eye. The final mean value of the parameters for each eye was obtained by taking the average of the values found by the same two researchers.
Statistical analyses of the data were performed using IBM SPSS statistics software version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed in terms of count (n) and percent (%). Normality was checked for each continuous variable using the Kolmogorov–Smirnov test. Pearson’s chi-square test was used to compare the categorical variables within the three groups. One-way analysis of variance (ANOVA) test was used to compare the continuous variables within the three groups, and post hoc analyses were performed with a Bonferroni adjustment for the multiple comparisons. Pearson’s correlation coefficient was used for the analyses of correlations. A p value of <0.05 was considered statistically significant.
Results
The mean time between the recruitment of the post-COVID-19 patients for the study and the confirmed negative COVID-19 RT-PCR test of the patients was 3.75 ± 2.33 months (a 2–10 month range) in Group 1 and 4.25 ± 1.36 months (a 1–10 month range) in Group 2 (p = 0.402). Except for one patient, all the post-COVID-19 patients were treated and followed up without being hospitalised. In Group 1, five patients (21%) reported that they had complaints about conjunctival hyperaemia, one patient stated (4%) having epiphora, and one patient reported having diplopia (4%) during COVID-19. Also, one patient (4%) mentioned having conjunctival hyperaemia, and one patient (4%) reported eye pain in Group 2. The neurological manifestations in Group 1 were loss of taste and smell (n = 20, 83%), peripheral neuropathy and myopathy (n = 8, 33%), and abducens nerve paralysis (n = 1, 4%).
The demographic characteristics of the groups are shown in Table 1. There were no significant differences in age or gender among the groups (p = 0.550 and p = 0.204). One-way ANOVA test revealed statistically significant differences in the mean NFL, NFD, and NBD values between the three groups (p < 0.001 for all). In the post hoc analyses, it was found that the mean NFD values were significantly lower in all post-COVID-19 patients (Group 1 and Group 2) compared to the control group (p < 0.001, p < 0.001, Table 2 and Fig. 2). Also, the mean NFD values of Group 1 were significantly lower than those of Group 2 (p = 0.011, Table 2). The mean NBD value of Group 1 was significantly lower compared to both Group 2 and Group 3 (p < 0.001 and p < 0.001, Table 2), but there was no significant difference in terms of NBD value between Group 2 and Group 3 (p = 0.445, Table 2). As well, the mean NFL values of Group 1 were significantly lower compared to both Group 2 and Group 3 (p < 0.001 and p < 0.001, Table 2), but there was no significant difference in terms of NFL value between Group 2 and Group 3 (p = 0.085, Table 2).
The value of the mean corneal sensitivity was significantly higher in group 3 compared to group 1 and group 2 (p < 0.001 and p < 0.001, Table 2) but there was no significant difference in terms of the value of the mean corneal sensitivity between group 1 and group 2 (p = 1.000, Table 2). Correlation analysis revealed a mild to moderate, statistically significant positive correlation between corneal sensitivity and NFL (r = 0.346, p = 0.005), NFD (r = 0.463, p < 0.001), and NBD (r = 0.322, p = 0.010).
Discussion
Many studies on COVID-19 patients with cranial neuropathies have been recently published [4,5,6,7]. For instance, in a review by Mehraeen et al. the authors studied 24 published articles about COVID-19 patients with olfactory and gustatory dysfunctions [3]. In this review, it was shown that COVID-19 patients had anosmia in the vast majority of the studies (95.8%), and this symptom has been accepted as a possible early sign of SARS-CoV-2 infection [3]. In the study of Kirschenbaum et al. autopsies were performed on two patients who died due to COVID-19. It was noted that one patient had a loss of taste and smell at presentation [4]. In this postmortem study, it was shown in the histological analysis the occurrence of axonal damage and inflammation in the olfactory bulbs of the patients.
Furthermore, a 36-year-old COVID-19 patient with abducens and oculomotor nerve palsy and a 71-year-old COVID-19 patient with abducens nerve palsy were reported by Dinkin et al. [5]. It was reported that ophthalmoparesis started in these two patients after the initial symptoms, including cough, fever, and myalgia. Also, a 69-year-old COVID-19 patient with bilateral trochlear nerve palsy was documented by Oliveira et al. [6]. Binocular diplopia started in this patient after the onset of complaints, such as fever, cough, and pain. In addition, Ferreira et al. published a young COVID-19 case with trigeminal neuropathy who had herpes zoster co-infection [7]. The authors commented that SARS-CoV-2 probably triggered the reactivation of the varicella-zoster virus and caused the involvement of the trigeminal nerve divisions in this patient. As summarised in the studies mentioned above, SARS-CoV-2 has an affinity for nerve tissues.
In general, the COVID-19 diagnosis was confirmed when the nasopharyngeal swab samples of the patients were positive for SARS-CoV-2 on RT-PCR. In the studies published to date, it has been shown that not all patients with a positive RT-PCR COVID-19 result had ocular symptoms [8]. Also, the RT-PCR results of conjunctival swab samples of all COVID-19 patients with ocular symptoms were not positive [14]. For instance, Wu et al. conducted a study with 38 COVID-19 patients, and the authors found that 12 of them had ocular manifestations, including conjunctivitis, epiphora, or chemosis [8]. In addition, the authors reported that the results for SARS-CoV-2 on RT-PCR from conjunctival swabs were positive in only two of the 12 COVID-19 patients. In a recent paper, postmortem COVID-19 patients were investigated, and the authors determined SARS-CoV-2 genomic RNA in the corneas of six out of 11 deceased patients with COVID-19 [15]. Positive results for SARS-CoV-2 RNA from conjunctival swab samples were detected in four out of six corneas with positive SARS-CoV-2 genomic RNA and in one out of five remaining postmortem COVID-19 patients who had positive results for SARS-CoV-2 RNA from the conjunctival swab in the study by Casagrande et al. [15].
The possible mechanisms between COVID-19 and ocular involvement are still unclear, but some hypotheses have been proposed. Angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease type 2 (TMPRSS2) are thought to be cellular receptors for SARS-CoV-2 and are responsible for the cellular sensitivity of the virus [16]. It is known that ACE2 and TMPRSS2 are known to be expressed in endothelial cells and neurons [17]. Also, ACE2 and TMPRSS2 have been found in the conjunctival, limbal, and corneal epithelium cells [18]. This fact may explain why the ocular findings occur in COVID-19 patients. Therefore, it has been reported that the ocular surface is responsible for both SARS-CoV-2 infection and SARS-CoV-2 transmission [19]. Many studies have aimed to evaluate the effect of SARS-CoV-2 infection on the ocular surface, but there is still a lot of information to learn, although most of the studies in the literature have reported the ocular symptoms, findings, and ocular involvement rates of COVID-19 patients. Still, as far as it is seen, the number of studies examining the effects of SARS-CoV-2 on ocular structures is relatively small in the literature, and investigating the impact of SARS-CoV-2 on the cornea is one of the strengths of the this study.
The cornea is the most innervated tissue in the body, and corneal innervation is provided by the ophthalmic division of the trigeminal nerve. The nerve departs into many branches before entering the cornea through the limbus. Small nerve branches that enter the cornea radially from the middle stroma and parallel to the corneal surface move forward as they branch. Finally, it forms the sub-basal nerve plexus in the sub-Bowman layer, which densely innervates the central cornea, creating corneal sensitivity [20]. Real-time, high-resolution images at the cellular level can be obtained with IVCM. This non-invasive method makes it possible to perform quantitative, qualitative, and morphometric analyses in the living cornea [11]. With the use of IVCM, it has been demonstrated that many ocular or systemic diseases, such as ocular infections, neurotrophic keratopathy, herpes simplex keratitis, diabetes mellitus, and multiple sclerosis, affect CSNP and impair corneal sensitivity [21,22,23,24]. The diseases mentioned above also cause an inflammatory process in the cornea. Indeed, animal studies have shown an association between corneal inflammation and corneal innervation [25, 26]. In addition, corneal esthesiometry widely used to assess corneal sensitivity and this technique allows the evaluation of the function of CSNP [27]. In the study of Zemaitiene et al. patients with herpes simplex virus keratitis and uveitis have been investigated. There, it has been reported that the herpes simplex virus causes a more significant reduction in the parameters of CSNP, including NFD and NBD, in the affected eyes than in the fellow eyes of the same patients [10]. Also, a significant decrease in the mean values of corneal sensation has been shown in the affected eyes compared to fellow eyes. Additionally, the CSNP and corneal sensitivity of acute or chronic Herpes Simplex Keratitis (HSK) patients were evaluated in a study by Hamrah et al. [21]. The authors classified HSK patients according to the grade of corneal sensitivity measured using a Cochet-Bonnet esthesiometer. It was reported that 19% of HSK patients had normal corneal sensation, 39% had mild hypaesthesia, and 42% had severe hypaesthesia. Predictably, it was found that the mean sub-basal nerve density, total nerves, and total branches in the corneas of HSK patients with mild or severe hypaesthesia were significantly lower than those of controls. But it was demonstrated that the same parameters were markedly lower in HSK patients with normal corneal sensation than controls. The notable outcome of this study was that morphological changes in CSNP probably are not enough to impact corneal sensitivity in some HSK patients. Similarly, the morphological changes in the CSNP of post-COVID-19 patients may not clinically affect corneal sensation in some cases.
This study was aimed to investigate the morphological changes quantitatively in the CSNP of post-COVID-19 patients by IVCM. With the outcomes of our research, it was revealed that the mean NFD, NBD, and NFL values of post-COVID-19 patients with neurological manifestations were significantly lower than those of both post-COVID-19 patients without neurological manifestations and controls. Except for the mean NFD value, there were no significant differences in the mean NBD and NFL values between the control group and the post-COVID-19 patients without neurological manifestations. Additionally, it was detected that the mean corneal sensitivity values were significantly lower in all post-COVID-19 patients compared to controls and there was no difference in this parameter between post-COVID-19 patients with and without neurological manifestations.
Single-centre study design, small sample size, no correlations with functional tests, and no follow-up of the patients are the limitations of this study. We should state that it is unknown whether these structural changes in the CSNP are reversible, definitive, or progressive. As far as is known, anosmia often improves over time in many post-COVID-19 patients. A similar recovery in the CSNP of post-COVID-19 patients may occur eventually. Therefore, the studies with long-term follow-up of patients may reveal the morphologic changes in the CSNP of post-COVID-19 patients more clearly. To the best of our knowledge, the present study is one of the first studies aimed to research the effect of SARS-CoV-2 on CSNP in the ophthalmic literature. In our study, a different perspective on the ocular manifestation of COVID-19 was shown and discussed.
Conclusion
Although most post-COVID-19 patients included in the study did not have ocular symptoms, SARS-CoV-2 appeared to cause subclinical morphologic changes in the CSNP of the post-COVID-19 patients. Indeed, this outcome was more pronounced in post-COVID-19 patients with different neurological involvements. The preliminary results of this study showed that SARS-CoV-2 could probably affect many groups of neurons nonspecifically after it enters the human body. The underlying mechanism of neuron involvement in COVID-19 patients is still not clearly understood. The majority of the presented theories are still hypotheses. Many inflammatory mediators and biochemical cascades may be responsible for this condition. Therefore, more extensive clinical investigations should be conducted to obtain more detailed results.
Summary
What was known before
-
Most of the studies in the literature have reported the ocular symptoms, findings, and ocular involvement rates of COVID-19 patients. The possible mechanisms between COVID-19 and ocular involvement are still unclear.
What this study adds
-
The present study is one of the first studies aimed to research the effect of SARS-CoV-2 on corneal sub-basal nerve plexus in the ophthalmic literature. In our study, a different perspective on the ocular manifestation of COVID-19 was shown and discussed.
References
World Health Organization (2020) WHO Director—General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-mediabriefing-on-2019-ncov-on-11-february-2020. Accessed 2 Mar 2021.
Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41:3039–56. https://doi.org/10.1007/s10072-020-04708-8.
Mehraeen E, Behnezhad F, Salehi MA, Noori T, Harandi H, SeyedAlinaghi S. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence. Eur Arch Otorhinolaryngol. 2021;278:307–12. https://doi.org/10.1007/s00405-020-06120-6.
Kirschenbaum D, Imbach LL, Ulrich S, Rushing EJ, Keller E, Reimann RR, et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. https://doi.org/10.1016/S0140-6736(20)31525-7.
Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–3. https://doi.org/10.1212/WNL.0000000000009700.
Oliveira RMC, Santos DH, Olivetti BC, Takhashi JT. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arq Neuropsiquiatr. 2020;78:385–6. https://doi.org/10.1590/0004-282X20200052.
Ferreira ACAF, Romão TT, Macedo YS, Pupe C, Nascimento OJM, Fellow of the American Academy of Neurology (FAAN). COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol 2020;27:1748–50. https://doi.org/10.1111/ene.14361.
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–8. https://doi.org/10.1001/jamaophthalmol.2020.1291.
Daruich A, Martin D, Bremond-Gignac D. Ocular manifestation as first sign of Coronavirus Disease 2019 (COVID-19): interest of telemedicine during the pandemic context. J Fr Ophtalmol. 2020;43:389–91. https://doi.org/10.1016/j.jfo.2020.04.002.
Zemaitiene R, Rakauskiene M, Danileviciene V, Use V, Kriauciuniene L, Zaliuniene D. Corneal esthesiometry and sub-basal nerves morphological changes in herpes simplex virus keratitis/uveitis patients. Int J Ophthalmol. 2019;12:407–11. https://doi.org/10.18240/ijo.2019.03.09.
Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2010;29:30–58. https://doi.org/10.1016/j.preteyeres.2009.11.001.
Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–8. https://doi.org/10.1007/s00125-003-1086-8.
Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060–70. https://doi.org/10.1212/WNL.0000000000009937.
Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258:1351–2. https://doi.org/10.1007/s00417-020-04695-8.
Casagrande M, Fitzek A, Spitzer MS, Püschel K, Glatzel M, Krasemann S, et al. Presence of SARS-CoV-2 RNA in the cornea of viremic patients with COVID-19. JAMA Ophthalmol. 2021;139:383–8. https://doi.org/10.1001/jamaophthalmol.2020.6339.
Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–44. https://doi.org/10.1016/j.jtos.2020.06.007.
Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020;27:1764–73. https://doi.org/10.1111/ene.14277.
Roehrich H, Yuan C, Hou JH. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020;39:1556–62. https://doi.org/10.1097/ICO.0000000000002509.
Napoli PE, Nioi M, d’Aloja E, Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual ‘ocular route’ exist? J Clin Med. 2020;9:1269. https://doi.org/10.3390/jcm9051269.
Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–42. https://doi.org/10.1016/s0014-4835(03)00050-2.
Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–6. https://doi.org/10.1016/j.ophtha.2010.07.010.
De Cillà S, Ranno S, Carini E, Fogagnolo P, Ceresara G, Orzalesi N, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Investig Ophthalmol Vis Sci. 2009;50:5155–8. https://doi.org/10.1167/iovs.09-3384.
Liu CY, Arteaga AC, Fung SE, Cortina MS, Leyngold IM, Aakalu VK. Corneal neurotization for neurotrophic keratopathy: Review of surgical techniques and outcomes. Ocul Surf. 2021;20:163–72. https://doi.org/10.1016/j.jtos.2021.02.010.
Fernandes D, Luís M, Cardigos J, Xavier C, Alves M, Papoila AL, et al. Corneal subbasal nerve plexus evaluation by in vivo confocal microscopy in multiple sclerosis: a potential new biomarker. Curr Eye Res. 2021:1–8. https://doi.org/10.1080/02713683.2021.1904509.
Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig Ophthalmol Vis Sci. 2012;53:867–72. https://doi.org/10.1167/iovs.11-8438.
Ferrari G, Chauhan SK, Ueno H, Nallasamy N, Gandolfi S, Borges L, et al. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Investig Ophthalmol Vis Sci. 2011;52:2532–9. https://doi.org/10.1167/iovs.10-5688.
Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp Eye Res. 2011;92:408–13. https://doi.org/10.1016/j.exer.2011.02.016.
Author information
Authors and Affiliations
Contributions
EM contributed to the concept and design and drafted the paper. EM, SB, AOG, and RO were involved in data acquisition and data analysis/interpretation. EM, SB, and MA contributed to statistical analysis. EM, SB, and RO were involved in the critical revision of the paper and supervision. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirza, E., Belviranli, S., Gundogan, A.O. et al. Quantitative assessment of the effect of SARS-CoV-2 on the corneal sub-basal nerve plexus of post-COVID-19 patients using in vivo confocal microscopy. Eye 37, 660–664 (2023). https://doi.org/10.1038/s41433-022-02018-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02018-1