Abstract
Background
To investigate the morphological features of corneal subbasal nerve plexus (CSNP) in normal-tension glaucoma (NTG) and primary open-angle glaucoma (POAG).
Methods
Thirty-four eyes with NTG (16 untreated), 23 eyes with POAG (11 untreated) and 31 eyes of healthy subjects were recruited. CSNP were assessed by corneal confocal microscopy (CCM) and peripapillary retinal nerve fibre layer (RNFL) was measured with optical coherence tomography (OCT). CCM parameters including corneal subbasal nerve fibre length (FL), corneal subbasal nerve branch number (BN), corneal subbasal nerve width (NW), corneal subbasal nerve reflectivity (NR), total and local corneal subbasal nerve tortuosity (NT) was compared across all groups, as well as between the topical medication treated and the nontreated patients.
Results
The newly diagnosed NTG patients had the longest FL (3619.15 ± 501.55), most BN (21.02 ± 5.90), thinnest corneal subbasal nerve width (3.04 ± 0.82), corneal subbasal nerve lowest reflectivity (140.43 ± 10.24) and the corneal subbasal nerves were most bending (1.09 ± 0.06) and tortuous (123.36 ± 7.82) compared with untreated POAG patients and controls. Untreated POAG had similar CSNP to controls. The treated glaucoma patients had longer FL and more BN than the nontreated but with no significant difference. FL and BN had correlations with RNFL thickness in untreated NTG patients, and NR and NW had correlations with RNFL thickness in untreated POAG patients. NT had no correlations with RNFL thickness.
Conclusions
The NTG group had different CSNP characteristics from the POAG group and controls, while the latter two shared more morphological features. The CCM parameters except NT had associations with the RNFL thickness in glaucoma patients.
Similar content being viewed by others
Introduction
Primary open angle glaucoma (POAG) is a multifactorial optic neuropathy, characterized by progressive thinning of retinal nerve fibre layer (RNFL) and visual field defect which could cause irreversible blindness [1]. Nearly 67 million people have the disease worldwide and the population is estimated to increase to 112 million by 2040 [1, 2]. Elevated intraocular pressure (IOP) is a prominent risk factor for POAG progression and is the main target for treatment [3]. Accumulative exposure to high IOP impairs retinal ganglion cells (RGC) whose axons constitute the RNFL and optic projection to the brain [4].
However, POAG’s association with IOP is not exclusive. A large number of POAG patients have an IOP within normal range and are categorized as normal tension glaucoma (NTG) [5]. NTG is a subtype of POAG manifesting RNFL defect and an open anterior chamber angle. NTG is more prevalent among Asian populations and its epidemiology varies [6]. Since its normal tension-independent feature and unique disease course, a controversy exists that whether it should be regarded as a disease within POAG spectrum or as a distinctive neurodegenerative disease [7]. The presence of typical glaucomatous optic nerve damage in NTG but with no clinical evidence of elevated IOP indicates other risk factors such as hemodynamic insufficiency [8,9,10] and immunologic factors [11].
Amid all of these efforts for exploring NTG path mechanisms, diffuse brain damage in NTG has been noticed in recent decade [12]. A high coincidence between NTG and progressive sensorineural hearing loss as well as an association of NTG and dysgeusia have also been reported [13, 14]. Neurodegenerative changes beyond retina and optic nerve head suggest possible pathogenesis of IOP-independent primary neural alterations of multiple tissues in NTG.
However, most of the knowledge about NTG neuropathy beyond the eyeball has been derived from nerve functional analysis. Structural observation is limited due to the invisible features of these nerves in vivo. The transparent nature of cornea allows morphologic evaluation of the corneal nerves in vivo by the means of corneal confocal microscopy (CCM). The cornea is densely innervated by the ophthalmic division of the fifth cranial nerve, the trigeminal nerve. The corneal subbasal nerve fibre plexus (CSNP) which lies between basal epithelium and Bowman’s membrane is the ending branch of ophthalmic nerve and shares the characteristics of small fibre nerves. The CSNP can be well studied by CCM and its degeneration has been discovered in many systemic neurodegenerative diseases such as Parkinson’s disease, amyotrophic lateral sclerosis and diabetic peripheral neuropathy where CSNP alteration was associated with other nerve system degeneration [15,16,17,18,19]. The visibility of CSNP by CCM has gained growing popularity in research as a disease severity predictor for neurological disorders.
With CCM, we examined the CSNP of NTG and POAG patients (with elevated IOP) and compared the structural changes of corneal nerves and then we explored the correlations between these anatomic alterations of corneal nerves and the glaucomatous changes of RNFL which is the most widely used parameter in clinical practice for measuring glaucoma progression. This study may add new knowledge to the current understanding of POAG and NTG neuropathy.
Materials and methods
This prospective observational study consecutively recruited 57 eyes of 30 glaucoma patients (34 eyes with NTG, 59.65%; 23 eyes with POAG,40.35%) seen in the Ophthalmology department of Peking University Third Hospital, Beijing, China from June 2017 to December 2019. It is important to note that the diagnosis for both eyes of each participant in our study was consistent. Thus, no patients were included in both NTG and POAG group. Twelve eyes (52.17%) of the POAG were under one to two kinds of antiglaucoma topical medication and the rest eleven (47.83%) were newly diagnosed without treatment of any eye drops. In particular, sixteen eyes (47.06%) of the NTG were newly diagnosed and untreated with any topical drugs. Eighteen eyes (52.94%) were under one to two kinds of antiglaucoma topical medication. The POAG and NTG were diagnosed by Glaucoma specialists. The diagnosis for POAG was based on the characteristic glaucomatous optic neuropathy, two reliable visual field tests with repeatable glaucomatous defect, open-angle on gonioscopy and IOP more than 21 mmHg on two consecutive visits using a Goldman applanation tonometer. The diagnosis for NTG was based on the same criteria with IOP lower than 21 mmHg. Inclusion criteria were: more than 18 years old, diagnosis of POAG or NTG, willing to participate and being able to finish the CCM examination. Exclusion criteria were: secondary open-angle glaucoma, angle closure, any corneal diseases like inflammation, leucoma and pterygium, any retinal diseases like retinal atrophy, bleeding or oedema, and Diabetes and other central neurologic disorders (Parkinson’s disease, amyotrophic lateral sclerosis et al.) with known corneal nerve alterations. Thirty-one eyes of 18 healthy control subjects who presented at the hospital for routine examination were enrolled as control group. The control group had no eye diseases other than low refractive error (myopia lower than 2D and astigmatism lower than 1D), and with ages more than 18 years old. Written consent was obtained from all participants, and the study was approved by the Ethical Committee of Peking University Third hospital. Investigations were conducted in accordance with the tenets of the Declaration of Helsinki.
All participants underwent a complete ophthalmic examination including a detailed slit lamp evaluation of the anterior segment, retinoscopy, CCM (Heidelberg Retina TomographIII Rostock Cornea Module, Heidelberg, Germany), visual field (Octopus 900, HAAG-STREIT international) and OCT (Zeiss Cirrus HD-OCT, Model 4000, Carl Zeiss Meditec, Inc).
Following previously published procedures, CSNP around the central cornea was scanned with CCM at the depth of 35-50μm using the sequence mode and the working area of 400×400μm. Each eye was anesthetized by 0.4% Oxybuprocaine drops before examination and during the procedure the participants were asked to keep sitting position and fixate on a target light to assure scanning central corneas. A disposable sterile cap was set on the objective lens and ophthalmic gel was applied as a coupling medium between the lens cap and the cornea. At least 10 images of CSNP were obtained for each subject and 3–5 high quality images with no overlapping were selected for analysis. The scans were all performed by one experienced operator masked for patient’s history and grouping [20].
The CSNP was quantitatively analysed using Neuron J software (an image J plugin, available online at image J.net). The assessment included: corneal subbasal nerve fibre length (FL), corneal subbasal nerve branch number (BN), corneal subbasal nerve fibre width (NW), corneal subbasal nerve fibre reflectivity (NR), and corneal subbasal nerve tortuosity (NT). The corneal subbasal nerve FL was defined as the total length of all nerve fibres and branches per image indicating nerve density per image. Corneal subbasal nerve BN was defined as the total number of branches emanating from major nerve trunks per image. Corneal subbasal nerve NW was the width of the major nerve trunk calculated as a mean of 9 measurements for each image. Corneal subbasal nerve NR was the average brightness of the nerve fibre per image which could be automatically acquired by the software. NT was measured by two methods. Local NT was defined as the local angle the nerve trunk formed and was calculated as the average of 9 measurements for each image depicting the smoothness of the nerve. Total NT was defined as the ratio of the nerve trunk length to the straight distance between the two ends of it which indicated the bending of the nerve, and was also calculated as the average of three measurements for each image. Each parameter was a mean value of all 3-5 images selected for every eye.
Mean deviation (MD) was obtained in visual field examination and was recorded as different stages according to the previously published staging system [21]. Corrected vision acuity was examined before visual field test.
Peripapillary RNFL thickness was measured for the POAG, NTG and controls by OCT. Optic nerve head was scanned to determine the RNFL thickness map. The temporal, superior, nasal and inferior quadrants of peripapillary RNFL were analysed.
Statistical analysis was carried out using SPSS (Statistical Package for Social Sciences) Windows version 20.0. Data were presented as mean±SD. The differences among the three groups were tested with analysis of variance (ANOVA) for age and with Chisquare for sex. Differences in ophthalmological measures (FL, NB, NW, NR and NT) between two groups were assessed with independent-samples T Test. The correlations of CSNP parameters with RNFL thickness in all groups and with medication duration in the POAG group were evaluated using Spearman correlation coefficient. The p value less than 0.05 was considered statistically significant.
Results
Patient characteristics
The demographic data and clinical characteristics of all participants are presented in Table 1. There were no significant differences in mean age and gender distribution among the NTG, POAG and control groups (P = 0.476 for age and P = 0.075 for gender). Eleven eyes of POAG and 16 eyes of NTG were newly diagnosed and had not started medicine treatment when enrolled, and they did not have significant difference in age and gender distribution either (P = 0.178 for age and P = 0.580 for gender). Neither did the POAG patients with and without treatment (P = 0.220 for age and P = 0.469 for gender). The NTG patients with and without treatment had no significant difference in age (P = 0.171) but the gender distribution showed difference (P = 0.002). The medication time for the POAG patients and NTG patients (prostaglandin analogues only or combined with beta-blockers) ranged from 5 to 50 months with an average of 20.67 ± 16.36 months and 5 to 156 months with an average of 60.11 ± 41.80 respectively (P = 0.003). MD stage recorded in median and quartile was used to evaluate disease severity and there was no significant difference between the NTG and POAG group (p = 0.138), between untreated NTG and untreated POAG (p = 0.682) or patients with or without medication (p = 0.350 in POAG, p = 0.146 in NTG).
Comparison of the CCM parameters of CSNP among the POAG, the NTG and the controls
Firstly, we analysed all patients including treated and untreated as well as the healthy subjects.
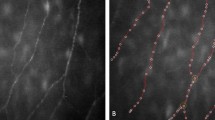
The NTG group had the longest nerve FL of 3651.54 ± 627.28 μm while it was 3156.79 ± 643.92 μm in the POAG and 3223.78 ± 617.06 μm in the controls. The difference between NTG and POAG was significant (p = 0.005), and so was the difference between NTG and control (p = 0.007). The difference between POAG and controls was not significant (p = 0.699). NTG also had the most BN (21.97 ± 7.51), and the control group (13.38 ± 5.44) had the least. The difference among the groups was significant (Table 2). The POAG had the most NV but the difference among the groups were not significant. The POAG also had the highest NR of 153.09 ± 14.12, much brighter than NTG (140.25 ± 12.12) and the controls (143.91 ± 15.91) (p = 0.001, and p = 0.020 respectively). The NTG had the highest total NT (1.08 ± 0.04) and the lowest local NT (126.88 ± 7.79) indicating the bending and tortuous feature of the corneal nerve in NTG. The control subjects had the lowest total NT (1.06 ± 0.03) and the highest local NT (132.07 ± 11.00) suggesting a relatively straight direction feature of the nerve. The total and local NT of POAG (1.07 ± 0.03 and 130.56 ± 13.03) were in the middle of the NTG and control (Table 2, Fig. 1). The difference among the groups was not significant.
Secondly, we analysed each CNSP parameters among the untreated NTG, untreated POAG patients (both marked with m-) and the control to exclude the potential impact of antiglaucoma eye drops.
The NTG m− group had the longest nerve FL of all three groups with a length of 3619.15 ± 501.55 μm, while it was 3105.14 ± 705.45 μm in the POAG m− and 3223.78 ± 617.06 μm in the controls. The differences between NTG m− and POAG m− and NTG m− and control were 0.035and 0.038 respectively. The difference between POAG m− and control was not significant (p = 0.579). NTG m− group also had more BN (21.02 ± 5.90) than the other two groups, with control group the lowest (13.38 ± 5.44, p = 0.000) and POAG m− (18.28 ± 3.60, p = 0.073) in middle. The POAG m− had the thickest nerve fibre (3.73 ± 0.84) and the NTG m− had the thinnest NW of 3.04 ± 0.82. The difference between NTG m− and POAG m− was significant (p = 0.026). The POAG m− also had the highest NR of 150.87 ± 14.42, while the NTG m− had the lowest NR of 140.43 ± 10.24, but the difference was not significant among the three. The NTG m− had the highest total NT (1.09 ± 0.06) and the lowest local NT (123.36 ± 7.82) suggesting the bending and tortuous nerve morphology. The POAG m− showed similar total (p = 0.924) and local (p = 0.799) NT to the control (Table 2).
From the above results, the CSNP of NTG group could be described as dense bending and tortuous nerves with many branches and lower reflectivity. The POAG had CSNP more similar to that of the control group, but with more nerve width and reflectivity.
Comparison of CCM parameters of CSNP in treated and untreated patients
We analysed each CSNP parameters between the treated (marked as m+) and untreated (marked as m-) eyes in both POAG and NTG groups. No significant difference was detected between POAG m+ and m− in corneal nerve length, width, reflectivity and branch number (Table 2). The NTG m+ and m- had similar results but NTG m+ had more nerve width (p = 0.000) and local tortuosity (p = 0.011) than the NTG m−.
Correlation between CCM parameters and medication duration
The correlation between each CCM parameters and the duration of anti-glaucoma medicine treatment in the POAG patients was analysed and no significant association could be seen (Table 3). In NTG group a significant correlation was seen between medication time and the nerve width (Table 3).
Correlation analysis between CCM parameters and OCT findings in the POAG, the NTG and the controls
The correlation of peripapillary RNFL thickness and the corneal nerve parameters were analysed in the POAG, NTG and the control groups.
NT had no correlations with RNFL thickness in any group. FL and BN had correlations with RNFL thickness in NTG m− group (r = 0.738, p = 0.037 and r = −0.714, p = 0.047 respectively), but not correlated with POAG m− group. NR had negative correlation with RNFL thickness in POAG patients and untreated POAG patients. NR also had negative correlation with RNFL thickness in the control. However, NR had positive correlations with RNFL thickness in NTG group.
In POAG group, the nerve FL was associated with the superior quadrant of RNFL thickness (r = 0.547, p = 0.043). Both the superior and nasal quadrants of RNFL thickness were statistically correlated with nerve BN. (nasal: r = 0.568, P = 0.034; superior: r = 0.535, P = 0.049). The brightness of nerve (NR) had significant correlations with all but the nasal quadrant of RNFL thickness (Table 4). The NW and NT were not associated with the thickness of peripapillary RNFL in POAG group. Both NW and NR were correlated with inferior and nasal quadrant RNFL thickness in NTG group (r > 0.7, p < 0.05).
As for untreated patients, in POAG m- group, NR had negative correlation with inferior quadrant thickness (r = −0.738, p = 0.037) and NW had negative correlation with nasal quadrant thickness (r = −0.783, p = 0.022). In NTG m− group, both BN and NW were negatively correlated with superior quadrant thickness and FL was correlated with inferior quadrant thickness (Table 4). Temporal thickness had no correlations with corneal nerve index in NTG m– and POAG m− groups.
Discussion
NTG has challenged the traditional pathophysiological viewpoints of glaucoma ever since it was recognized in 1900 [22]. The occurrence of RNFL thinning and optic nerve atrophy in the absence of elevated IOP aroused a presumption that NTG could be classified as a disease of the neural system as well as the eye [23]. Evidence has shown a close pathogenetic link between NTG and degenerative diseases of central neural system such as Parkinson’s disease and Alzheimer’s disease [24, 25]. Giorgio and colleagues [12] reported neurodegenerative findings across the brain in NTG. They suggested that glaucoma may be associated with central nervous system degeneration, independent of IOP. Despite central nerve system, dysfunction of peripheral nerves such as auditory nerve and olfactory nerve were also found in NTG patients [13, 14]. However, corneal nerve of NTG is rarely studied though both central and peripheral nerve damage in NTG have been reported [26, 27].
CSNP is a good representative of peripheral small nerve fibre [28]. Thanks to the transparent and richly innervated nature of the cornea, images of CSNP noninvasively captured by in vivo CCM is now widely used in studies regarding neuropathy. Evolving evidence suggests that the corneal nerve network might reflect the peripheral and central neurodegeneration. The corneal nerve fibre length and nerve branches which are the two most commonly used CCM parameters have been reported to be associated with disease severity in some neural disorders such as diabetic neuropathy, Parkinson’s disease and multiple sclerosis [29,30,31]. The corneal nerve in NTG group in our study also showed special nerve fibre length and nerve branches which were associated with RNFL thickness in untreated NTG patients.
The characteristic morphology of corneal nerves found in NTG patients in our research was quite different from the features seen in the POAG and the control providing additional information to NTG’s peripheral neural alterations. The NTG group showed the densest (longest nerve length per image) and most bending corneal nerves with more branches and lower reflectivity compared with POAG and the controls. While the corneal nerve in POAG was wider and brighter with less nerve length (density) and less nerve branches. The POAG and the control group had similar NT which was straighter than the NTG. To exclude the potential impact of antiglaucoma eye drops, we also analyzed the newly diagnosed POAG and NTG patients who had similar visual field defect but had not received topical medication treatment. The results were the same as above. This provided supportive information that NTG might be a primary neurodegenerative disease different from POAG.
Corneal biomechanics and central corneal thickness were analysed in NTG but few investigations have been made on corneal nerve in NTG. [32, 33] Would the specific nerve pattern of NTG shown in our research raise the question whether primary corneal nerve alternations maybe a feature of NTG, independent of IOP? A whorl-like corneal subbasal nerve plexus in control eyes was depicted by Utsunomiya’s report which was in the inferocentral area of corneas [29]. However, the bending feature observed in our NTG patients was not whorl like (Fig. 1) but a zigzag pattern and the area we examined was in the centre instead of the inferocentre. So, the winding nerves seen in our result was not because of incorrect location nor a shift of nerve distribution. It was more likely a primary alteration in NTG.
Our findings indicated that the corneal nerve parameters of POAG lay in the middle between NTG and controls and were more similar to the controls especially in untreated POAG. Ranno and colleagues also found similar corneal confocal findings between control subjects and untreated POAG glaucoma patients and concluded that their untreated glaucoma patients and controls had similar corneal parameters [34].
Our limited knowledge of how the topical antihypertension medication affects corneal nerve. Ranno compared nontreated POAG patients and those on medical treatment for at least two years and came up with a result that both corneal nerve tortuosity and reflectivity in treated patients were lower than in nontreated patients [34]. Martone found a decrease of CSNP number and an increase of nerve tortuosity in medication treated POAG compared with the controls [35]. Baratz concluded that chronic therapy with glaucoma medications leads to decline of corneal subbasal fibre number and density [36]. Our research showed a longer FL, more BN, thinner NW, higher NR, and more bending nerves in treated POAG than untreated patients but the difference was not significant. And no significant correlation was found between medication time and the CCM parameters in POAG patients. The explanation might be that in Ranno’s research patients should use the antiglaucoma eye drops for at least two years but in our research the medication duration is much less. Long-standing medical glaucoma treatment may cause corneal nerve alteration [32]. In our study, treated NTG patients had longer medication duration (60.11 ± 41.80 months) than the POAG, and they had less tortuous but thicker corneal nerves than nontreated NTG patients. Long-time use of antiglaucoma eye drops might cause corneal nerve alteration. More participants should be included for further confirmation.
We also analysed the correlations between RNFL and the CCM parameters and found that they had some associations. Corneal nerve parameters such as nerve branches, reflectivity, nerve length and width in glaucoma patients were associated with RNFL thickness, but the nerve tortuosity was not associated with retinal changes. The temporal quadrant thickness had the least correlation with the CCM parameters.
The limitations of the current study were the small number of subjects and the lack of tear film status records as well as the functional evaluations of the peripheral nerves. As for the effect of the anti-glaucoma topical medication on corneal nerves in glaucoma patients, a well-designed study is in need to differentiate the process of neurodegeneration and the drug’s impact. Moreover, some participants had both eyes enrolled in this study. Through, there was no necessary consistency as to corneal nerve morphology in one’s paired eyes, we would choose one eye for further analysis.
Our study showed that the corneal nerves in NTG patients have characteristic pattern different from that in the POAG and the controls, while the latter two share more morphological features. And the nerve parameters in CCM have associations with the RNFL thickness, a marker for disease progression in those glaucoma patients. The results added supportive information to the pathological link between NTG and the neural system alteration. Also, it provoked the re-thinking of NTG’s entity adscription. Antiglaucoma topical medications might have an impact on corneal nerve morphology after a long time use, but it should be further distinguished from a probable primary corneal neurodegeneration in glaucoma patients.
In conclusion, the NTG patients had unique nerve morphology in CSNP which was quite different from that in the POAG and the controls, while the latter two had more common features. The CCM parameters might be potential indicators for glaucoma prognosis as they were associated with the RNFL thickness.
Summary
What was known before
-
Previous studies have demonstrated that NTG have associated both central and peripheral nerve damage.
What this study adds
-
NTG have unique nerve morphology in CSNP which might be a primary neurodegenerative disease different from POAG.
-
Discrepancy Between Normal Tension Glaucoma and Primary Open Angle Glaucoma with Confocal Microscopy Examination of Corneal Nerves.
References
Faralli JA, Filla MS, Peters DM. Role of fibronectin in primary open angle glaucoma. Cells-Basel. 2019;8:1518.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–90.
Risner ML, McGrady NR, Pasini S, Lambert WS, Calkins DJ. Elevated ocular pressure reduces voltage-gated sodium channel NaV1.2 protein expression in retinal ganglion cell axons. Exp Eye Res. 2020;190:107873.
Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–19.
Kim KE, Park KH. Update on the prevalence, etiology, diagnosis, and monitoring of normal-tension glaucoma. Asia Pac. J Ophthalmol (Philos). 2016;5:23–31.
Trivli A, Koliarakis I, Terzidou C, Goulielmos GN, Siganos CS, Spandidos DA, et al. Normal-tension glaucoma: pathogenesis and genetics. Exp Ther Med. 2019;17:563–74.
Mastropasqua R, Fasanella V, Agnifili L, Fresina M, Staso SD, Gregorio AD, et al. Advance in the pathogenesis and treatment of normal-tension glaucoma. Prog Brain Res. 2015;221:213–32.
Chiotoroiu SM, Stefaniu O, Noaghi M, Teodorescu A, Taina L. The role of systemic blood pressure in glaucoma progression. Rom J Ophthalmol. 2015;59:141–7.
Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed Res Int. 2015;2015:141905.
Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharm. 2013;13:43–9.
Rizzo MI, Greco A, De Virgilio A, Gallo A, Taverniti L, Fusconi M, et al. Glaucoma: recent advances in the involvement of autoimmunity. Immunol Res. 2017;65:207–17.
Giorgio A, Zhang J, Costantino F, De Stefano N, Frezzotti P. Diffuse brain damage in normal tension glaucoma. Hum Brain Mapp. 2018;39:532–41.
Kremmer S, Kreuzfelder E, Bachor E, Jahnke K, Selbach JM, Seidahmadi S. Coincidence of normal tension glaucoma, progressive sensorineural hearing loss, and elevated antiphosphatidylserine antibodies. Br J Ophthalmol. 2004;88:1259–62.
Omoti AE, Edema OT. A review of the risk factors in primary open angle glaucoma. Niger J Clin Pr. 2007;10:79–82.
Aksoy D, Ortak H, Kurt S, Cevik E, Cevik B. Central corneal thickness and its relationship to Parkinson’s disease severity. Can J Ophthalmol. 2014;49:152–6.
Hubers A, Muller HP, Dreyhaupt J, Böhm K, Lauda F, Tumani H, et al. Retinal involvement in amyotrophic lateral sclerosis: a study with optical coherence tomography and diffusion tensor imaging. J Neural Transm (Vienna). 2016;123:281–7.
Zis P, Grunewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci. 2017;378:204–9.
Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk factors associated with corneal nerve alteration in Type 1 Diabetes in the absence of neuropathy: a Longitudinal in vivo corneal confocal microscopy study. Cornea 2016;35:847–52.
Liu Z, Wang H, Fan D, Wang W. Comparison of optical coherence tomography findings and visual field changes in patients with primary open-angle glaucoma and amyotrophic lateral sclerosis. J Clin Neurosci. 2018;48:233–7.
Villani E, Sacchi M, Magnani F, Nicodemo A, EI Wiliams S, Rossi A, et al. The ocular surface in medically controlled glaucoma: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2016;57:1003–10.
Mills RP, Budenz DL, Lee PP, Noecker RJ, Walt JG, Siegartel LR, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24–30.
Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond). 2018;32:924–30.
Zhang HJ, Mi XS, So KF. Normal tension glaucoma: from the brain to the eye or the inverse? Neural Regen Res. 2019;14:1845–50.
Eraslan M, Cerman E, Cekic O, Balci S, Dericioglu V, Sahin O, et al. Neurodegeneration in ocular and central nervous systems: optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk J Med Sci. 2015;45:1106–14.
Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology 2012;119:2161–7.
Lee JW, Wong RL, Chan JC, Wong IY, Lai JS. Differences in corneal parameters between normal tension glaucoma and primary open-angle glaucoma. Int Ophthalmol. 2015;35:67–72.
Artero-Castro A, Rodriguez-Jimenez FJ, Jendelova P, VanderWall KB, Meyer JS, Erceg S. Glaucoma as a neurodegenerative disease caused by intrinsic vulnerability factors. Prog Neurobiol. 2020;193:101817.
Khan A, Petropoulos IN, Ponirakis G, Menzies RA, Chidiac O, Pasquier J, et al. Corneal confocal microscopy detects severe small fiber neuropathy in diabetic patients with Charcot neuroarthropathy. J Diabetes Investig. 2018;9:1167–72.
Utsunomiya T, Nagaoka T, Hanada K, Omae T, Yokota H, Abiko A, et al. Imaging of the corneal subbasal whorl-like nerve plexus: more accurate depiction of the extent of corneal nerve damage in patients with Diabetes. Invest Ophthalmol Vis Sci. 2015;56:5417–23.
Mikolajczak J, Zimmermann H, Kheirkhah A, Kadas EM, Oberwahrenbrock T, Muller R, et al. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult Scler. 2017;23:1847–53.
Kass-Iliyya L, Javed S, Gosal D, Kobylecki C, Marshall A, Petropoulos LN, et al. Small fiber neuropathy in Parkinson’s disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord. 2015;21:1454–60.
Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45:11–8.
Park K, Shin J, Lee J. Relationship between corneal biomechanical properties and structural biomarkers in patients with normal-tension glaucoma: a retrospective study. Bmc Ophthalmol. 2018;18:7.
Ranno S, Fogagnolo P, Rossetti L, Orzalesi N, Nucci P. Changes in corneal parameters at confocal microscopy in treated glaucoma patients. Clin Ophthalmol. 2011;5:1037–42.
Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–35.
Baratz KH, Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea 2006;25:1046–52.
Acknowledgements
Authors’ contributions: ZY Liu and DL Jing: interpretation of data and drafting the work, YL Chou, HK Wang and S Gao: acquisition of data, W Wang and X Fan: design of the work and revising the work. All authors have read and approved the manuscript.
Dr. H Zhang is a statistician working in Peking University third hospital and helped us with statistical consultation for this manuscript.
Funding
This study was partly sponsored by the National Natural Science Fund of China (Grant Number: 81900826).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jing, D., Liu, Z., Chou, Y. et al. Discrepancy between NTG and POAG with corneal nerves in CCM. Eye 36, 1662–1668 (2022). https://doi.org/10.1038/s41433-021-01705-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01705-9