Abstract
Purpose
To investigate the choroidal vascularity index (CVI) in patients affected by leptochoroid.
Methods
Three distinct age-matched cohorts were collected: patients with reticular pseudodrusen (RPD) secondary to age-related macular degeneration, patients with high-myopia, and healthy controls. CVI was calculated in the subfoveal 6000 μm diameter area.
Results
54 eyes (54 patients) were included (18 eyes in each cohort). No statistical differences were disclosed in terms of age between controls, RPD patients (p = 0.062), and myopic patients (p = 0.070). Total choroidal area showed a different distribution among the 3 cohorts (p < 0.001), due to the reduction of luminal and stromal choroidal area in both RPD and myopic groups in comparison to controls (p < 0.001). Interestingly, CVI showed a different distribution between the 3 cohorts (p < 0.001). In detail, RPD group showed no changes in CVI in comparison to controls (p = 1.000), whereas the myopic group showed a higher CVI in comparison to both RPD group and controls (p < 0.001 in both analyses).
Conclusions
Different changes of the choroidal vascular and stromal components characterize the leptochoroid secondary to RPD eyes and high-myopic eyes. The relative greater impairment of the vascular area in RPD eyes in comparison to myopic eyes could be at the basis of the lower development of RPD in patients with high myopia.
Similar content being viewed by others
Introduction
“Leptochoroid” is defined as a choroidal thinning that characterizes several macular diseases [1,2,3,4,5,6,7,8]. A thinning of the choroidal layers and a reduction of the choriocapillaris perfusion were previously reported in different stages of non-neovascular (or dry) and neovascular age-related macular degeneration (dAMD and nAMD, respectively) [1,2,3, 9,10,11]. Of note, patients affected by reticular pseudodrusen (RPD) secondary to dAMD showed a greater reduction of choroidal thickness in comparison to patients affected by typical drusen [12,13,14], suggesting that RPD may be the result of choroidal atrophy and ischemia leading to photoreceptor outer segments disruption and accumulation above the retinal pigment epithelium (RPE) [12]. On the other hand, also patients affected by high myopia (HM) were characterized by a severe leptochoroid, but a lower prevalence of AMD and RPD. For this reason, several groups have suggested a protective role of HM on the development of dAMD [15,16,17,18]. However, no studies have elucidated the differences in choroidal structures between these two diseases characterized by a leptochoroid (i.e., RPD and HM).
Recently, the introduction of a new structural optical coherence tomography (OCT) tool, namely choroidal vascularity index (CVI), has allowed us to better investigate the choroidal changes, especially in the ratio between stromal and luminal area [19]. Several studies reported CVI alterations in patients affected by dAMD [20,21,22]. However, to date, no studies examined the CVI alterations in leptochoroid associated with RPD in comparison to HM. The aim of this study is to investigate the CVI and other choroidal features in patients affected by RPD or HM featuring leptochoroid, in order to gather insights on the pathophysiology of these conditions. We selected RPD eyes due to the more severe degree of leptochoroid in this phenotype among intermediate AMD phenotypes [12,13,14].
Methods
In this retrospective cohort study, patients affected by RPD secondary to dAMD (i.e., RPD group) or HM (i.e. myopic group) were collected in the Medical Retina & Imaging Unit of the Department of Ophthalmology of University Vita-Salute San Raffaele in Milan, Italy, between January 2020 and June 2020. The study was conducted in agreement with the Declaration of Helsinki for research involving human subjects. All subjects signed informed consent for retrospective studies that were approved by the Ethics Committee of San Raffaele Institute.
The present study consists of three distinct cohorts: RPD group, myopic group, and a group of healthy age-matched subjects (i.e., control group).
Inclusion criteria for the RPD group were: (1) age greater than 50 years, (2) diagnosis of RPD secondary to dry AMD (3) clear ocular media to ensure proper image quality. Exclusion criteria for the RPD group were: (1) presence of any retinal disorder other than AMD (e.g., diabetic retinopathy, retinal vein or artery occlusion, posterior uveitis), (2) presence of other AMD phenotype rather than RPD, (3) presence of MNV, (4) any previous retinal treatments (e.g., intravitreal injections, photodynamic therapy), (5) myopia greater than 6 dioptres (D) of sphere or 3D of the cylinder, and/or axial length >25.5 mm.
Inclusion criteria for the high myopic group were: (1) age greater than 50 years, (2) diagnosis of HM (defined as an axial length >25.5 mm or a refractive error > −6 D of spherical equivalent) [4], (3) clear ocular media to ensure proper image quality. Exclusion criteria for the myopic group were: (1) presence of any retinal disorder other than myopia (e.g., AMD, diabetic retinopathy, retinal vein or artery occlusion, posterior uveitis), (2) any previous retinal treatments (e.g., intravitreal injections, photodynamic therapy), (3) presence of myopic MNV.
Inclusion criteria for the control group were: (1) age greater than 50 years, (2) clear ocular media to ensure proper image quality. We excluded subjects with any retinal disorder and/or myopia greater than 6D of sphere or 3D of cylinder, and/or axial length >25.5 mm
If both eyes presented inclusion criteria, only one eye was included. The eye with the higher quality of structural OCT image was selected as the study eye. If both eyes have the same quality of OCT image, the included eye was selected by flipping a coin.
Clinical data of patients were recorded and reviewed. In detail, all patients underwent a complete ophthalmologic examination with a multimodal imaging approach as part of the standard clinical assessment of our Unit. We recorded best-corrected visual acuity (BCVA) using Snellen charts, infrared reflectance (IR), short-wave fundus autofluorescence (SW-FAF), structural spectral-domain OCT, and axial length measurement. All OCT examinations were performed using the enhanced depth imaging (EDI) OCT in order to achieve a good visualization of the choroid. OCT-angiography (OCT-A) and/or dye angiographies (i.e., fluorescein angiography [FA] and indocyanine green angiography [ICGA]) were also performed in case of clinical and multimodal imaging suspicion of MNV presence. IR, SW-FAF, structural OCT, FA, and ICGA were performed using the Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). OCT-A examinations were performed using PLEX® Elite 9000 (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Axial length was measured using a noncontact biometry instrument (IOLMaster, Carl Zeiss Meditec AG, Jena, Germany).
Imaging analysis
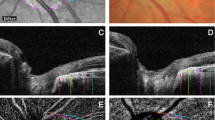
Subfoveal choroidal thickness (ChT), mean ChT, and central macular thickness (CMT) were recorded. Two trained examiners (EB and GV) measured the distance from the Bruch’s membrane to the sclerochoroidal interface in the subfoveal location (subfoveal ChT) and the mean value was used for statistical analyses. Furthermore, the ChT was also measured 500 and 1000 μm nasally and temporally to the fovea, and the mean value among the 5 measurements (subfoveal, 500 μm nasally, 1000 μm nasally, 500 μm temporally, and 1000 μm temporally to the fovea) was used in order to calculate the mean ChT. CMT was defined as the vertical distance from the Bruch’s membrane and vitreoretinal interface. CMT was recorded with the Spectralis software (Heidelberg Eye Explorer, Version 1.9.14.0, Heidelberg, Germany) in the central 1 mm-diameter circle.
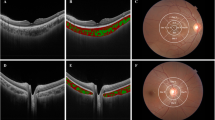
Horizontal and vertical B-scan of structural EDI OCT passing through the fovea were exported by the Spectralis Software. The choroidal parameters were analysed using a previously reported algorithm [23]. Briefly, the choroidal analysis involved automated binarization of an HD horizontal and vertical 6 mm OCT B-scan after defining the choroidal boundaries.
The Bruch-RPE junction and the sclerochoroidal junction were selected as the upper and lower boundary of the region of interest (ROI). Total choroidal area (TCA) was calculated as the total area of the ROI. Luminal choroidal area (LCA) was calculated as the dark pixels of the ROI after binarization. Stromal choroidal area (SCA) was calculated as the white pixels of the ROI after binarization. CVI was obtained as the ratio between LCA and TCA [23].
Statistical analysis
Statistical analyses were performed using SPSS Statistics Version 20 (IBM, Armonk, New York, USA). All values of descriptive analyses were expressed as means ± standard deviations for quantitative variables and as counts and percentages for categorical variables. In all patients, BCVA was converted to Logarithm of the Minimum Angle of Resolution (LogMAR) for statistical analysis. The agreement between individual measurements of subfoveal and mean ChT from both readers was performed using the intraclass correlation coefficient (ICC; 95% CI). Comparisons of BCVA, axial length, CMT, subfoveal ChT, mean ChT, TCA, LCA, SCA, and CVI between the 3 cohorts were performed using the Analysis of Variance (ANOVA) with Bonferroni post-hoc analysis. In all analyses, p-values <0.05 were considered statistically significant.
Results
Patient demographics and main clinical features
A total of 54 eyes of 54 Caucasian patients (18 eyes for each group) were included in the study. Thirty-nine patients were females and 15 patients were males. The mean age of all patients was 71.2 ± 8.6 years (median 73.5; range 50–83 years). In detail, the mean age of controls (71.3 ± 7.0 years) did not show significant differences in comparison to myopic patients (65.4 ± 9.6 years; p = 0.062) and to RPD patients (77.0 ± 4.4 years; p = 0.070).
BCVA showed significant differences among study cohorts (p = 0.041). In detail, mean BCVA was between 20/25 and 20/32 Snellen equivalent in myopic and RPD patients (0.17 ± 0.22 LogMAR and 0.15 ± 0.13 LogMAR respectively), and between 20/20 and 20/25 Snellen equivalent (0.05 ± 0.05 LogMAR) in controls. Furthermore, also the mean axial length was different among study cohorts (p < 0.001). Indeed, myopic patients showed a greater axial length (26.71 ± 1.42 mm, range 25.58–30.36) in comparison to RPD patients (23.73 ± 0.61 mm, range 22.70–24.87, p < 0.001) and controls (23.78 ± 0.51 mm, range 22.72–24.66, p < 0.001). Of note, no difference in axial length was disclosed between the RPD group and controls (p = 1.000).
All main clinical features of the whole population and of each cohort are reported in Table 1.
Choroidal analysis
Analysing ChT among the three different cohorts, subfoveal ChT and mean ChT showed significantly different values among study groups in both analyses (p < 0.001 in both analyses) (Table 1). Myopic patients showed a thinner subfoveal ChT and mean ChT in comparison to both the RPD group (p = 0.004 and p = 0.002, respectively) and controls (p < 0.001 in both analyses). Furthermore, RPD group showed a thinner subfoveal ChT and mean ChT in comparison to controls (p = 0.003 in both analyses). In detail, subfoveal ChT was 90 ± 84 μm, 187 ± 75 μm, and 286 ± 94 μm in myopic, RPD, and control groups, respectively, and 85 ± 83 μm, 179 ± 71 μm, and 270 ± 78 μm considering the mean ChT. Interobserver variability between readers was excellent for all measurements [ICC = 0.962 (0.927–0.997)].
All binarized images were analysed in order to obtain the LCA, SCA, TCA, and CVI. Following the ChT trend, mean TCA was lower in myopic patients in comparison to RPD patients (p = 0.025) and to controls (p < 0.001) (Table 2) (Figs. 1–3). Moreover, the RPD group showed a lower TCA in comparison to controls (p < 0.001). In the analysis of the vascular and stromal choroidal area, the RPD group showed a lower value of both LCA and SCA in comparison to controls (p = 0.001 and p < 0.001, respectively) whereas, interestingly, no differences were disclosed considering the CVI (p = 1.000) (Figs. 1 and 3). On the other hand, the myopic group showed a lower value of both LCA and SCA in comparison to controls (p < 0.001 in both analyses) but a greater CVI in comparison to controls (p < 0.001) (Table 2) (Figs. 2 and 3). In other words, the SCA was greater impaired in comparison to LCA, resulting in an increased CVI.
Structural b-scan OCT passing through the fovea (A) was binarized after defining the choroidal boundaries (B). A region of interest (ROI) of 6000 μm centred to the fovea was selected (C). The Bruch-RPE junction and the sclerochoroidal junction were selected as the upper and lower boundary of the region of the ROI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Structural b-scan OCT passing through the fovea (A) was binarized after defining the choroidal boundaries (B). A region of interest (ROI) of 6000 μm centred to the fovea was selected (C). The Bruch-RPE junction and the sclerochoroidal junction were selected as the upper and lower boundary of the region of the ROI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Structural b-scan OCT passing through the fovea (A) was binarized after defining the choroidal boundaries (B). A region of interest (ROI) of 6000 μm centred to the fovea was selected (C). The Bruch-RPE junction and the sclerochoroidal junction were selected as the upper and lower boundary of the region of the ROI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Finally, comparing the RPD group with the myopic group, the latter showed a significantly lower SCA (p = 0.003) with a greater CVI (p < 0.001). All choroidal analyses were reported in Table 2.
Discussion
In this study, using the CVI analysis, we have disclosed that the vascular choroidal area is impaired to a greater extent in the leptochoroid of RPD patients compared with HM patients.
The thinning of the choroid (i.e., leptochoroid) was previously reported in several diseases, including high myopia and different stages of dAMD [1,2,3,4]. Of note, the impairment of the choroidal thickness was greater in AMD patients affected by RPD in comparison to AMD patients affected by drusen [12,13,14]. On the basis of this finding, several groups supported the hypothesis that the progressive choroidal atrophy and vascular impairment led to the damage of photoreceptors, disruption of the photoreceptor outer segments, resulting in the accumulation of the waste materials above the RPE (i.e., development of RPD) [12,13,14]. This hypothesis was also supported by other authors that demonstrated a reduction of the perfusion density of the choriocapillaris below RPD [24, 25]. However, the reduction of the choroidal thickness could not be the only driver into the pathogenesis of RPD. Indeed, in another macular disease characterized by a severe leptochoroid, namely HM, the prevalence of RPD is lower in comparison to patients without HM, suggesting a protective role of HM in the development of dAMD [15,16,17,18]. Storey et al. [26]. reported that amblyopic eyes due to anisometropia manifest decreased severity of AMD compared with non-amblyopic eyes in the same patient, supporting the protective role of high myopia in the development of dAMD. Furthermore, our group has displayed that myopic anisometropia manifests decreased rate of RPD presence compared with non-ambylopic eyes in the same patient (unpublished data).
In the last years, CVI has been proposed as a novel OCT-based parameter to quantify choroidal structural changes in different disorders, including AMD [19,20,21,22]. CVI allows disclosing in vivo specific alterations of the stromal and vascular area of the choroid, gathering insight into the pathogenesis of different diseases. In detail, several groups reported a reduced CVI in patients affected by iAMD and by GA [21, 22]. Velaga et al. [21] reported that CVI and other choroidal parameters are altered in patients affected by iAMD eyes in comparison to controls. However, no studies have investigated and compared alterations of CVI in different diseases characterized by leptochoroid. Here, we compared, face to face, two cohorts of patients affected by leptochoroid (i.e., RPD and myopic groups), in order to disclose the possible different status of vascular and stromal areas of the choroid characterizing these two diseases. Based on our results, we disclosed a significant reduction of both vascular and stromal areas (i.e., LCA and SCA) in RPD and myopic groups in comparison to controls (p = 0.001 or p < 0.001 in all analyses). Interestingly, patients affected by high myopia showed a greater mean value of the ratio between vascular area and total choroidal area (i.e., CVI) in comparison to controls (p < 0.001) and to the RPD group (p < 0.001). In other words, although both RPD and HM eyes are characterized by a thinning of the choroid (p < 0.001 in the analyses of subfoveal and mean ChT in comparison to age-matched controls), myopic eyes showed a lower relative impairment of the vascular area in comparison to eyes affected by RPD secondary to AMD, resulting in an increased CVI. Our findings could explain why patients affected by HM have a lower prevalence of RPD in comparison to patients without HM (i.e., protective role of HM on the development of dAMD). The greater impairment of LCA in RPD eyes in comparison to HM eyes could be of paramount importance in the pathogenesis of RPD development. Indeed, although a thinning of the choroid using structural OCT was reported on the basis of the RPD [12,13,14], we hypothesize that the main risk factor should be considered the reduction of the vascular area of the choroid due to the stromal replacement and not the entire ChT. Our hypothesis is supported by previously reported histological and in vivo data. Of note, McLeod et al. [27] reported that changes of choroidal interstitial stroma characterize the development of AMD in comparison to healthy patients analysing 11 post-mortem eyes. Furthermore, using in vivo imaging analysis, our group and others described that choroidal cytoarchitecture is profoundly altered in the setting of AMD changes, with an altered ratio between the vascular component and the fibrous matrix [9, 28, 29]. In this setting, the fibrous matrix increases while the vascular component gradually reduces, inducing a different distribution of the choroidal vessels in iAMD patients [9]. Of note, these phenomena are greater in RPD patients among dAMD phenotypes, resulting in unique choroidal re-arrangements that take place in these patients [9].
Drawbacks of our study should be disclosed, mainly due to the relatively small sample size and the retrospective nature. Furthermore, we have not included a group with concomitant RPD and HM, but the prevalence of RPD in HM patients is uncommon. One can argue that we have not included patients with drusen secondary to dAMD. However, patients affected by drusen are not characterized by leptochoroid and it was yet reported that drusen eyes showed no CVI differences in comparison with healthy eyes [21].
Another possible limitation of the study is that, potentially, in thicker choroids, the different choroidal vascular layers (choriocapillaris, Sattler’s, and Haller’s) are better graphically represented and this could be a bias in CVI evaluation comparing HM patients and patients affected by RPD. Indeed, in HM patients there is a greater loss of choriocapillaris and Sattler’s layer in comparison to patients affected by RPD. However, a subanalysis of CVI separately for Sattler’s and Haller’s area could not be performed with reliability due to the alterations of the choroidal architecture in HM and RPD patients. Furthermore, it was demonstrated that CVI is not influenced by the ChT in healthy patients [30, 31].
On the other hand, also the strengths of this study should be kept in mind. First of all, we have compared three age-matched cohorts. Although Zhou et al. [30]. reported that CVI remained constant in all regions with age, matching the age of patients of the different cohorts allowed us to compare not only the CVI but also the ChT and the total volume of the choroid. Indeed, it is well known that ChT and the volume of the choroid are influenced by the age of patients. Secondly, the CVI analysis could be influenced by several factors. For this reason, we have used a previously reported and validated algorithm [23].
In conclusion, we have disclosed that CVI is a more sensitive biomarker in detecting choroidal changes associated with leptochoroid as compared to ChT. Using CVI, we have highlighted the difference in the stromal and vascular impairment in two different diseases characterized by leptochoroid (i.e., RPD and HM), providing insights for better understanding the pathogenesis of these diseases. The greater relative impairment of the vascular area in RPD eyes in comparison with HM could be on the basis of the lower incidence of RPD in HM eyes.
Summary Table
What was known before
-
No studies investigated choroidal features in leptochoroid.
What this study adds
-
In this study, reporting choroidal features in leptochoroid secondary to pseudodrusen in comparison to high-myopia, we proposed that relative lower vessel impairment in myopic eyes could explain the protective role of myopia on pseudodrusen development.
References
Yiu G, Chiu SJ, Petrou PA, Stinnett S, Sarin N, Farsiu S, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159:617–26.
Capuano V, Souied EH, Miere A, Jung C, Costanzo E, Querques G. Choroidal maps in non-exudative age-related macular degeneration. Br J Ophthalmol. 2016;100:677–82.
Govetto A, Sarraf D, Figueroa MS, Pierro L, Ippolito M, Risser G, et al. Choroidal thickness in non-neovascular versus neovascular age-related macular degeneration: A fellow eye comparative study. Br J Ophthalmol. 2017;101:764–9.
Corbelli E, Parravano M, Sacconi R, Sarraf D, Yu SY, Kim K, et al. Prevalence and phenotypes of age-related macular degeneration in eyes with high myopia. Invest Ophthalmol Vis Sci. 2019;60:1394–402.
Canestraro J, Jaben KA, Wolchok JD, Abramson DH, Francis JH. Progressive choroidal thinning (leptochoroid) and fundus depigmentation associated with checkpoint inhibitors. Am J Ophthalmol Case Rep. 2020;19:100799.
Guo Y, Liu L, Zheng D, Duan J, Wang Y, Jonas JB, et al. Prevalence and associations of fundus tessellation among junior students from greater Beijing. Invest Ophthalmol Vis Sci. 2019;60:4033–40.
Shen JH, Chen KJ, Wang NK. Leptochoroid in a case of Alagille syndrome (Arteriohepatic Dysplasia). Ophthalmology. 2017;124:1135.
Yan YN, Wang YX, Xu L, Xu J, Wei WB, Jonas JB. Fundus tessellation: Prevalence and associated factors: The Beijing Eye Study 2011. Ophthalmology. 2015;122:1873–80.
Sacconi R, Cicinelli MV, Borrelli E, Savastano MC, Rispoli M, Lumbroso B. Haller’s vessels patterns in non-neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2020;258:2163–71.
Sacconi R, Corbelli E, Carnevali A, Querques L, Bandello F, Querques G. Optical coherence tomography angiography in geographic atrophy. Retina. 2018;38:2350–5.
Sacconi R, Corbelli E, Borrelli E, Capone L, Carnevali A, Gelormini F, et al. Choriocapillaris flow impairment could predict the enlargement of geographic atrophy lesion. Br J Ophthalmol. 2021;105:97–102.
Querques G, Querques L, Forte R, Massamba N, Coscas F, Souied EH. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012;53:1258–63.
Mrejen S, Spaide RF. The relationship between pseudodrusen and choroidal thickness. Retina. 2014;34:1560–6.
Rabiolo A, Sacconi R, Cicinelli MV, Querques L, Bandello F, Querques G. Spotlight on reticular pseudodrusen. Clin Ophthalmol. 2017;11:1707–18.
Pan CW, Cheung CY, Aung T, Cheung CM, Zheng YF, Wu RY, et al. Differential associations of myopia with major age-related eye diseases: The Singapore Indian eye study. Ophthalmology. 2013;120:284–91.
Curtin B, Karlin D. Axial length measurements and fundus changes of the myopic eye. Am J Ophthalmol. 1971;71:42–53.
Böker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol. 1993;2:10–13.
Pallikaris IG, Kymionis GD, Ginis HS. Ocular rigidity in patients with age-related macular degeneration. Am J Ophthalmol. 2006;141:611–5.
Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, et al. Choroidal Vascularity Index: An in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. 2020;9:595.
Wei X, Ting DSW, Ng WY, Khandelwal N, Agrawal R, Cheung CMG. Choroidal vascularity index - a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. 2017;37:1120–5.
Velaga SB, Nittala MG, Vupparaboina KK, Jana S, Chhablani J, Haines J, et al. Choroidal vascularity index and choroidal thickness in eyes with reticular pseudodrusen. Retina. 2020;40:612–7.
Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Moscardelli F, Iovino C, et al. Choroidal vascularity index quantification in geographic atrophy using binarization of enhanced-depth imaging optical coherence tomographic scans. Retina. 2020;40:960–5.
Vupparaboina KK, Nizampatnam S, Chhablani J, Richhariya A, Jana S. Automated estimation of choroidal thickness distribution and volume based on OCT images of posterior visual section. Comput Med Imaging Graph. 2015;46:315–27.
Cicinelli MV, Rabiolo A, Sacconi R, Lamanna F, Querques L, Bandello F, et al. Retinal vascular alterations in reticular pseudodrusen with and without outer retinal atrophy assessed by optical coherence tomography angiography. Br J Ophthalmol. 2018;102:1192–8.
Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2017;174:42–55.
Storey PP, Aziz HA, O’Keefe GAD, Borchert M, Lam LA, Puliafito CA, et al. Decreased severity of age-related macular degeneration in amblyopic eyes. Br J Ophthalmol. 2018;102:1575–8.
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–91.
Zheng F, Gregori G, Schaal KB, Legarreta AD, Miller AR, Roisman L, et al. Choroidal thickness and choroidal vessel density in nonexudative age-related macular degeneration using swept-source optical coherence tomography imaging. Invest Ophthalmol Vis Sci. 2016;57:6256–64.
Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–64.
Zhou H, Dai Y, Shi Y, Russell JF, Lyu C, Noorikolouri J, et al. Age-related changes in choroidal thickness and the volume of vessels and stroma using swept-source OCT and fully automated algorithms. Ophthalmol Retin. 2020;4:204–15.
Breher K, Terry L, Bower T, Wahl S. Choroidal biomarkers: A repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl Vis Sci Technol. 2020;9:8.
Funding
GV, SB, and MB: none. KKV: None. JC: Allergan Inc (Irvine, California, USA), Biogen (Cambridge, Massachusetts, USA), Salutaris, OD-OS. RS is a consultant for: Novartis (Basel, Switzerland), and Zeiss (Dublin, USA). EB is a consultant for: Novartis (Basel, Switzerland), and Zeiss (Dublin, USA). FB is a consultant for Alcon (Fort Worth,Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). GQ is a consultant for Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Amgen (Thousand Oaks, USA), Heidelberg (Germany), KBH (Chengdu, China), LEH Pharma (London, UK), Lumithera (Poulsbo, USA), Novartis (Basel, Switzerland), Bayer Shering-Pharma (Berlin, Germany), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Soof-Fidia (Albano, Italy), Zeiss (Dublin, USA).
Author information
Authors and Affiliations
Contributions
RS, EB: research design, data acquisition, and analysis, interpretation of data, drafting the paper, and critical revision of the paper. SB, GV, MB, and KKV: data acquisition and analysis, critical revision of the paper. JC, FB, and GQ: interpretation of data and critical revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sacconi, R., Borrelli, E., Balasubramanian, S. et al. Choroidal vascularity index in leptochoroid: A comparative analysis between reticular pseudodrusen and high myopia. Eye 37, 75–81 (2023). https://doi.org/10.1038/s41433-021-01889-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01889-0
This article is cited by
-
Choroidal remodeling following different anti-VEGF therapies in neovascular AMD
Scientific Reports (2024)