Abstract
Background/Objectives
We investigated the effect of blepharoptosis on refractive errors across different age groups in Korean population.
Subjects/Methods
This cross-sectional study was performed with data obtained in the Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2012. A total of 33,103 participants were enroled in our study. Blepharoptosis was defined as a marginal reflex distance 1 (MRD1) less than 2 mm, and was diagnosed in 3,305 (9.98%) participants. Ophthalmic examinations were performed, including measurements of MRD1, spherical equivalent, and degree of astigmatism. The age range was divided into three groups: less than 20 years old; more than 20 years and less than 60 years old; and more than 60 years old.
Results
The mean spherical equivalent were −0.28 ± 2.23 D in the ptotic eyelids and −1.13 ± 2.30 D in the non-ptotic eyelids (p < 0.001, 95% CI: −0.93, −0.77). The mean cylinder dioptre were −1.03 ± 0.87 D and −0.80 ± 0.77 D respectively (p < 0.001, 95% CI: 0.20, 0.26). The association with the eyelid position and refractive error significantly differed according to the age group and body mass index. Increased positive spherical change and increased astigmatism were prominent among ptotic participants aged less than 60 years.
Conclusions
A decrease in MRD1 was associated with a hyperopic shift and higher astigmatism. Mechanical compression of the ptotic eyelid may affect ocular biometry, with the effect being particularly prominent in younger participants who had greater eyelid tension.
Similar content being viewed by others
Introduction
Blepharoptosis refers to the drooping of the eyelid and is a common ophthalmic disorder. Depending on the time of occurrence, it is categorized as congenital or acquired. It is often caused by aponeurotic degeneration and presents with age-related changes. Blepharoptosis causes functional problems such as obscuring of the superior visual field, but it also causes cosmetic and psychological distress. In children with congenital blepharoptosis, it can lead to ophthalmic conditions such as amblyopia, strabismus, or astigmatism [1,2,3].
Blepharoptosis is usually accompanied by dermatochalasis, in which the cornea is covered with redundant skin and muscle. The eyelid that covers the external ocular surface can cause changes in keratometry values by applying pressure to the cornea [4,5,6,7].
Several investigators have reported refractive changes after eyelid surgeries such as blepharoptosis surgery or blepharoplasty by using corneal topography [4, 7, 8]. Patients who undergo eyelid surgery often complain of prolonged blurred vision postoperatively [9]. Blepharoptosis is defined as drooping of the eyelid that can contribute to corneal pressure because of continuous compression. Such changes may lead to corneal flattening and cause alterations of the refractive error or keratometry findings.
Although many previous articles have reported changes in refraction and keratometry values before and after surgery in blepharoptosis patients, to our knowledge, no studies have shown the differences in these values in relation to the presence of blepharoptosis. Various factors, such as age or body mass index (BMI), may affect blepharoptosis and refractive power. Individuals with blepharoptosis were older and were more likely to have high BMI [10, 11]. The present study aimed to report the association of refractive errors with blepharoptosis, and the moderating effect of age group on the association, by using nation-wide, population-based, cross-sectional data of the Korean National Health and Nutrition Examination Survey (KNHANES).
Materials/Subjects and methods
Korean National Health and Nutrition Examination Survey and Participants
This study used data from the KNHANES, which was performed in 2008–2012 by the Korean Centres for Disease Control and Prevention and the Korean Ministry of Health and Welfare, Sejong, Republic of Korea. The study design of the KNHANES has been previously described in detail [12, 13].
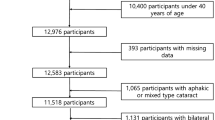
A total of 35,648 individuals in KNHANES-IV and V were identified as candidates for this study. Participants with a history of ophthalmic surgery, including double-eyelid surgery, refractive surgery, or cataract extraction, were excluded (n = 2545), and a total of 33,103 individuals were finally analysed.
All participants provided written consent before enrolment. The Institutional Review Board of the Korea Centres for Disease Control and Prevention (KCDC) reviewed and approved this nationally representative data study.
Eyelid measurements
Ophthalmic examinations were performed by well-trained ophthalmologists and residents. The eyelid position was indicated by MRD1 (marginal reflex distance 1) in millimetres, which was defined as the distance between the corneal reflex light in the primary gaze and the upper eyelid margin. When dermatochalasis compromised the eyelid margin, MRD1 was measured with only the skin lifted carefully to exclude the effect of sagging skin.
We subdivided MRD1 into five groups: MRD1 ≥ 4 mm; 3 mm ≤ MRD1 ≤ 3.9 mm; 2 mm ≤ MRD1 ≤ 2.9 mm; 1 mm ≤ MRD1 ≤ 1.9 mm; and MRD1 < 1 mm. Blepharoptosis was defined as an MRD1 of less than 2 mm.
Refraction
The refractive data were examined with an autorefractor-keratometer (KR8800; Topcon, Tokyo, Japan) under non-cycloplegic conditions. The refractive power was measured at intervals of 0.25 dioptre (D), and measurements were obtained three times and the average value was determined. The spherical power, cylindrical power, and spherical equivalent were measured. The spherical equivalent was calculated as follows: (spherical D) + (½×cylindrical D). Cylindrical power was recorded as (−). Astigmatic types were divided into 3 groups according to the most powerful meridian: with-the-rule (WTR) of 60–120 degrees; against-the-rule (ATR) of zero to 30 degrees or 150–180 degrees; oblique axis (OA) of 31–59 degrees or 121–149 degrees. Only data from the left eyes were selected for analysis.
Demographic variables
Participants were divided into three groups according to age: (1) <20 years, (2) 20 to <60 years, or (3) ≥60 years. BMI was calculated as body weight (kg)/heights2 (m2) and was classified into three categories as follows: (1) ≤18.5; (2) >18.5 to <25; or (3) ≥25.
Statistical analysis
Student t test was used for comparisons of the mean spherical equivalent and degree of astigmatism according to the classification variables. Chi-square tests with p for trend were used for statistical comparisons of astigmatic types according to the eyelid level. These data were analysed using a moderated regression model with the Hayes’ PROCESS macro for SPSS [14, 15]. Simple moderation analysis was used to determine whether the effect of MRD1 on spherical equivalent and astigmatism varied in magnitude and nature as a function of the age group. This analysis identified statistical interactions between variables that were predictors (MRD1) and variables that were moderators (age group), and the strength and direction of their effects on the outcome variable (spherical equivalent and astigmatism) after adjustment for sex and BMI. Regression analyses were also performed to assess the influence of MRD1 on spherical equivalent and astigmatism with moderation by age group.
Results
This study included 33,103 participants. Table 1 shows the differences in spherical equivalent by classification according to variables. The mean spherical equivalent was −1.11 ± 2.26 D for males and −1.09 ± 2.36 D for females, and the difference between sexes was not statistically significant. The age groups were distributed as follows: 7,492 participants were <20 years old, 18,116 were 20 to <60 years old, and 7495 were ≥60 years old. The mean spherical equivalent values in these three age groups were as follows: −1.82 ± 2.25 D, −1.44 ± 2.25 D, and +0.44 ± 1.79 D, respectively. The differences in refractive error across age groups were statistically significant, with the younger groups showing a greater myopic tendency. In ptotic participants (n = 3305, 9.98%), spherical equivalent was more obviously hyperopic (−0.28 ± 2.23 D) than that in non-ptotic individuals (−1.13 ± 2.30 D). Mean spherical equivalent showed more positive change with increasing BMI. The mean spherical equivalent was −1.39 ± 2.19 D in the group with BMI less than 18.5 kg/m2, −1.13 ± 2.36 D in the group with BMI between 18.5 and 25 kg/m2, and −0.91 ± 2.70 D in the group with BMI above 25 kg/m2, with the differences being significant (p < 0.01).
In assessment of astigmatism, the mean astigmatism were −0.76 ± 0.81 D, −0.71 ± 0.71 D, and −1.12 ± 0.85 D, respectively, in the three age groups. In ptotic eyes, astigmatism increased more than that in non-ptotic eyes (−0.79 ± 0.77 D, −1.03 ± 0.87 D), and the increase was statistically significant (p < 0.001). The degree of astigmatism increased with increasing BMI (Table 1).
Table 2 shows that astigmatic types were significantly different according to the severity of blepharoptosis. As MRD1 decreased, the proportion of WTR decreased and ATR and OA increased in a dose-response relationship (p for trend <0.001). Supplementary Table 1 shows the moderation model with the spherical equivalent as the dependent variable. The main effects of MRD1 and age group on the spherical equivalent were also statistically significant, as MRD1 decreased, the spherical equivalent showed more positive change (p < 0.01), and older age group had more positive spherical equivalent (p < 0.001). The interaction term indicates that the effect of MRD1 on the spherical equivalent is dependent on the age group. For example, the effect of MRD1 (1–1.9 mm) compared to MRD1 (≥ 4 mm) on the spherical equivalent observed in those younger than 20 years changed by –0.288 in those aged 60 years or older (interaction 6; p < 0.05). Thus, the effect of blepharoptosis on the spherical equivalent (tendency for increased positive change) observed in those younger than 20 year is counter-balanced or no longer present in those aged ≥60. BMI was associated with negative change in the spherical equivalent (p < 0.001). Sex was unrelated to the spherical equivalent (Supplementary Table 1).
Moderation analysis, controlled for the effects of sex and BMI, indicated that the effect of overall interaction between MRD1 and age group on the spherical equivalent was significant (R2 change = 0.0007, F = 3.124, p = 0.002).
Conditional effects of the predictor (MRD1) on the spherical equivalent at the values of the moderator (those aged < 20 years and those aged 20 to <60 years) showed more positive change as the MRD1 decreased. In contrast, among those aged over 60 years, MRD1 was unrelated to the spherical equivalent (Table 3, Fig. 1).
Supplementary Table 2 shows a moderation model with astigmatism as the dependent variable. The main effects of factors such as MRD1 and age group on astigmatism were also statistically significant, as MRD1 decreased, the degree of astigmatism increased (p < 0.01), and oldest age group (≥60 years) had increased degree of astigmatism (p < 0.001). The interaction term indicates that the effect of MRD1 on astigmatism is dependent on the age group (interactions 5 & 8). BMI was positively associated with the degree of astigmatism (p < 0.001). However, sex was unrelated to the spherical equivalent.
Moderation analysis, controlling for the effects of sex and BMI, indicated that the effect of the interaction between MRD1 and age group on astigmatism was significant (R2 change = 0.001, F = 4.840, p < 0.001).
The conditional effects of the predictor (MRD1) on astigmatism at all values of the moderator (all the age groups) revealed that the degree of astigmatism increased as the MRD1 decreased. In particular, the relationship was prominent among participants aged less than 60 years (Table 4, Fig. 2).
Discussion
The relationship of MRD1 with the spherical equivalent differed across the three age groups. Thus, for participants aged less than 20 years and those aged 20 to less than 60 years, the spherical equivalent showed more positive change as the MRD1 decreased. In contrast, for those aged 60 or older, MRD1 was unrelated to the spherical equivalent. We identified a hyperopic refractive shift in ptotic eyes in comparison with the non-ptotic eyes. In the groups with blepharoptosis, the refractive errors were in the direction of less myopia, which was more evident in the younger than the older group. The relationship of MRD1 with astigmatism also differed according to age group. Thus, for all age groups, the degree of astigmatism increased as the MRD1 decreased. In our study, astigmatism especially increased in ptotic eyes in participants aged less than 60 years. Our study identified that blepharoptosis also affected the axis of astigmatism. It caused a shift towards against-the-rule astigmatism and oblique astigmatism, which became more prominent as the MRD1 decreased.
Ptotic eyes showed a higher incidence of astigmatism and developed surgery-induced astigmatism after blepharoptosis surgery [5, 16]. Merriam et al. speculated that postoperative eyelid repositioning contributed to unexpected astigmatism, with non-significant astigmatism observed in the unoperated eye [16]. Cadera et al. reported that ptotic eyes showed a higher incidence of astigmatism as well as a tendency toward a postoperative increase in astigmatism [17]. Wilson et al. described that lid retraction using a lid speculum caused a shift to less with-the-rule astigmatism in comparison with eyelids in the normal position [18]. In addition, Knopf investigated the hyperopic refractive shift after blepharoptosis correction in some patients [19]. Langford et al. investigated asymmetric ocular development with elongation of the superior part in an animal model of congenital blepharoptosis [20]. However, Byard et al. did not observe a notable change in the refractive error after levator resection correction over an extended follow-up period of 36 months [21].
Some data suggest that the refractive error and keratometric values may be altered by the position of the upper eyelid, even if the alterations are temporary in most patients after blepharoptosis surgery [4, 7]. Gullstrand hypothesized that corneal astigmatism was altered in the with-the-rule direction by pressure on the eyelids, which was due to the peripheral flattening of the cornea from compression by the eyelids [22].
Several studies, such as those described above, have supported a mechanism dependent on changes in position of the eyelid in the operated eyes. Surgical repositioning of the eyelid exerts tension on the eyelid, which may increase the curvature and alter the contour of the corneal surface. Upper eyelid masses such as chalazions [23, 24] or haemangiomas [24] are associated with a higher incidence of astigmatic refraction. Several previous studies showing that surgical elevation of an eyelid causes a change in refractive errors support the assumption that mechanical compression or pressure changes as a result of the blepharoptosis can affect refractive errors by exerting pressure on the globe. Most prior studies have reported changes in the degree of astigmatism focused on after eyelid surgery. Our study focused on the relationship between refraction and long-standing blepharoptosis. We found changes in astigmatism as well as changes in the hyperopic shift of the spherical equivalent with a large-scale survey, and the changes appeared irrespective of eyelid surgery.
We hypothesized that the interaction between the eyelid and opposing corneal surface could be an important factor influencing the refractive errors. Indeed, the action of the eyelid/corneal interface can theoretically affect the refractive power, especially astigmatism and axis. The mechanical effect of the ptotic eyelid exerted pressure on the cornea and thus induced a hyperopic shift in the long term. Eyelid compression in blepharoptosis occurs across a larger corneal surface area and reduces with-the-rule astigmatism by flattening the cornea overall, increasing the degree of astigmatism, and decreasing myopia.
We also found that the refractive power shift to the hyperopic direction and higher astigmatism in ptotic eye was more prominent in groups with younger age and higher BMI. We intend to describe the explanation of the refractive change under the influence of the eyelid position with two theories: eyelid tension theory (age-related) and excess tissue theory (volume-related).
First, the tightness of the eyelid may affect the refractive power under mechanical forces. Eyelid laxity increased with the aging process. Loss of elasticity in the connective tissue of the eyelid may also contribute to a less compressive effect on the globe in the elderly population. On the other hand, younger individuals have tighter and more tense eyelids than older individuals, leading to increased pressure on the corneal surface, which causes corneal flattening and a hyperopic shift. This result may explain why the changes in refractive power were more noticeable in younger individuals with tighter eyelids than in older participants. Second, excess tissues such as skin and muscle can be expected to accentuate the effect of the eyelids on the ocular surface. Zinkernagel et al. reported that astigmatic changes were more evident after blepharoplasty with reduction of entire fat pads in comparison with cases of skin-only excisional blepharoplasty [6]. Wei et al. have shown that individuals with a higher BMI were more likely to have more hyperopic refractive power [25]. Such individuals tend to have thick and bulky eyelids, so more pronounced mechanical compression could be expected. These assumptions can explain why even individuals with a high BMI tend to show a shift to higher astigmatism with voluminous and fatty eyelids.
In the present study, the relationship between BMI and the spherical equivalent exhibited a tendency for positive change as the BMI increased in univariate analysis (Table 1), but a negative correlation in the multivariate analysis after adjustment for sex and age (Supplementary Table 1). These results in the multivariate analysis are considered to be an effect of age adjustment rather than sex. Several studies have shown that BMI was associated with refractive errors. Studies have reported associations between increased BMI and myopic refractions [26, 27], higher BMI and hyperopic refraction [25, 28], or have reported no association between BMI and refraction [29]. These contradictory results can occur because BMI is a systemic factor which can be affected by many diverse factors; therefore the results may be different for each studies. In addition, refraction is influenced by multiple environmental factors besides BMI, and the possibility of being affected by other factors that were not adjusted in this study cannot be overlooked [30]. BMI is a systemic factor associated with general obesity, so there may be a discrepancy with the local effect on the eyelid since it is not able to represent the entire eyelid volume. Regarding the relationship between BMI and astigmatism, however, the degree of astigmatism increased consistently as the BMI increased in both the univariate and multivariate analyses. The effect of blepharoptosis on astigmatism is less than that on the spherical equivalent.
This study had some limitations. First, the refractive error was measured using assessments of non-cycloplegic refraction in KNHANES, which may have resulted in inaccurate outcomes. However, the association of the refraction in ptotic eyes and non-ptotic eyes was consistent, indicating that this approach was not disadvantageous for large-scale research. Second, we did not obtain any keratometric values or corneal curvature measurements. Finally, MRD1 was not measured by one expert, but by several inspectors, which may have resulted in inter-observer variations. Despite these limitations, our study was designed on the basis of a nationwide Korean population-based survey, and we have provided considerable outcomes through a large-scale study. We evaluated whether more severe blepharoptosis is associated with a greater effect on refraction in a large population-based study.
In conclusion, less myopic eyes and high astigmatism were more likely to be encountered in individuals with blepharoptosis, with this effect dependent on the age group. Young people less than 60 years of age showed significant changes according to the position of the eyelid. The degree of astigmatism increased in participants with higher BMI. These results support the mechanical effect of the ptotic eyelid on the ocular surface.
Summary
What was known before
-
There were no prior large-scale population surveys about refraction differences in relation to blepharoptosis.
What this study adds
-
Hyperopic shift and higher astigmatism were significantly associated with blepharoptosis.
-
Mechanical compression of the ptotic eyelid can affect the ocular surface, resulting in refractive changes, which are more evident in individuals younger than 60 years due to increased eyelid tension.
-
The degree of astigmatism increased as MRD1 decreased, which was evident in individuals with higher BMI.
References
Hashemi H, Nabovati P, Dadbin N, Heidari Z, Yekta A, Jafarzadehpur E, et al. The prevalence of ptosis and its association with amblyopia and strabismus in. Strabismus. 2015;23:126–31.
Nemet AY, Segal O, Mimouni M, Vinker S. Associated morbidity of pediatric ptosis—a large, community based case-control. Graefes Arch Clin Exp Ophthalmol. 2014;252:1509–14.
Srinagesh V, Simon JW, Meyer DR, Zobal-Ratner J. The association of refractive error, strabismus, and amblyopia with congenital. J AAPOS. 2011;15:541–4.
Gingold MP, Ehlers WH, Rodgers IR, Hornblass A. Changes in refraction and keratometry after surgery for acquired ptosis. Ophthalmic Plast Reconstr Surg. 1994;10:241–6.
Klimek DL, Summers CG, Letson RD, Davitt BV. Change in refractive error after unilateral levator resection for congenital ptosis. J AAPOS. 2001;5:297–300.
Zinkernagel MS, Ebneter A, Ammann-Rauch D. Effect of upper eyelid surgery on corneal topography. Arch Ophthalmol. 2007;125:1610–2.
Kim YK, In JH, Jang SY. Changes in corneal curvature after upper eyelid surgery measured by corneal topography. J Craniofac Surg. 2016;27:e235–8.
Lee DH, Jung JH, Ahn JH. Changes in corneal curvature after epiblepharon surgery in children. J Craniofac Surg. 2018;29:e191–5.
Shao W, Byrne P, Harrison A, Nelson E, Hilger P. Persistent blurred vision after blepharoplasty and ptosis repair. Arch Facial Plast Surg. 2004;6:155–7.
Rha EY, Han K, Park Y, Yoo G. Socioeconomic disparities in the prevalence of blepharoptosis in the south korean adult population based on a nationwide cross-sectional study. PLoS One. 2016;11:e0145069.
Paik JS, Jung SK, Han KD, Kim SD, Park YM, Yang SW. Obesity as a potential risk factor for blepharoptosis: the Korea National Health and Nutrition Examination Survey 2008-10. PLoS One. 2015;10:e0131427.
Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43:69–77.
Yoon KC, Choi W, Lee HS, Kim SD, Kim SH, Kim CY, et al. An overview of ophthalmologic survey methodology in the 2008-15 Korean National Health and Nutrition Examination Surveys. Korean J Ophthalmol. 2015;29:359–67.
Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research. Behav Res Ther. 2017;98:39–57.
Hayes AF Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd edn. Guilford publications: New York; 2017.
Merriam WW, Ellis FD, Helveston EM. Congenital blepharoptosis, anisometropia, and amblyopia. Am J Ophthalmol. 1980;89:401–7.
Cadera W, Orton RB, Hakim O. Changes in astigmatism after surgery for congenital ptosis. J Pediatr Ophthalmol Strabismus. 1992;29:85–88.
Wilson G, Bell C, Chotai S. The effect of lifting the lids on corneal astigmatism. Am J Optom Physiol Opt. 1982;59:670–4.
Knopf H. Refractive distractions from drugs and disease. Ophthalmol Clin North Am. 1993;6:599–605.
Langford JD, Linberg JV, Blaylock WK, Chao GM. Axial myopia in congenital ptosis: an animal model. Ophthalmic Plast Reconstr Surg. 1998;14:261–5.
Byard SD, Sood V, Jones CA. Long-term refractive changes in children following ptosis surgery: a case series and a review of the literature. Int Ophthalmol. 2014;34:1303–7.
Gullstrand A. The cornea. Helmholtz’s Treatise Physiological Opt. 1962;1:320.
Ormond AW, NOTES ON, THREE, CASES OF. Acquired astigmatism associated with meibomian cysts. Br J Ophthalmol. 1921;5:117–8.
Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977;83:52–58.
Wei S, Sun Y, Li SM, Hu JP, Cao K, An W, et al. Effect of body stature on refraction and ocular biometry in Chinese young adults: The Anyang University Students Eye Study. Clin Exp Optom. 2021;104:201–6
Ye S, Liu S, Li W, Wang Q, Xi W, Zhang X. Associations between anthropometric indicators and both refraction and ocular. BMJ Open. 2019;9:e027212.
Kim H, Seo JS, Yoo WS, Kim GN, Kim RB, Chae JE, et al. Factors associated with myopia in Korean children: Korea National Health and. BMC Ophthalmol. 2020;20:31.
Saw SM, Chua WH, Hong CY, Wu HM, Chia KS, Stone RA, et al. Height and its relationship to refraction and biometry parameters in Singapore. Invest Ophthalmol Vis Sci. 2002;43:1408–13.
Badmus SA, Ajaiyeoba AI, Adegbehingbe BO, Onakpoya OH, Adeoye AO, Ameye SA. Relationship between refraction, anthropometrics, and educational status in a Nigerian Young Adult Population. Middle East Afr J Ophthalmol. 2018;25:30–34.
Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–49.
Author information
Authors and Affiliations
Contributions
YK and JHL had full access to all the data used in the study and take responsibility for the integrity of the data and the accuracy of the analysis; Study concept and design (YK and JHL); Acquisition, analysis, or interpretation of data (YK and JHL); Drafting of the manuscript (YK and JHL); Critical revision of the manuscript for important intellectual content (YK and JHL); Statistical analysis (YK); Study supervision (YK and JHL).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, Y., Lee, JH. Association of blepharoptosis with refractive error in the Korean general population. Eye 35, 3141–3146 (2021). https://doi.org/10.1038/s41433-021-01652-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01652-5
This article is cited by
-
Corneal biophysical changes after upper eyelid blepharoplasty and ptosis surgery: a review
BMC Ophthalmology (2023)