Abstract
Objectives
To comprehensively analyse the tear cytokine levels of patients with extranodal marginal zone B-cell lymphoma (EMZL) of the ocular adnexa (OA), and the association with clinical characteristics.
Methods
Tear cytokine concentrations of 21 OA-EMZL patients and 14 age- and sex-matched healthy controls were measured using a 27-multiplex bead analysis on a Luminex system. Tear break-up time, corneal fluorescent staining and other clinical and demographic data were collected as well. The diagnosis of OA-EMZL was established based on the incisional biopsy and histopathology.
Results
The concentrations of interleukin-1 receptor antagonist (IL-1RA) and IL-8, and the ratio of IL-1RA/IL-1β were significantly increased in OA-EMZL tear samples (all P < 0.05), while the levels of three cytokines (FGF-2, IL-2 and IL-4), as well as IL-10/IL-6 ratio were significantly decreased (all P < 0.05). The American Joint Committee on Cancer Tumour stage was significantly associated with tear concentrations of FGF-2 (r = −0.44, P = 0.043), GM-CSF (r = −0.49, P = 0.025) and IL-2 (r = −0.45, P = 0.042), while lacrimal gland lymphoma invasion was related to levels of IL-8 (r = 0.53, P = 0.012), FGF-2 (r = −0.43, P = 0.049) and IL-10/IL-6 ratio (r = −0.48, P = 0.026). Receiver operating characteristic (ROC) curve analysis revealed moderate diagnostic accuracy of these indices in differentiating OA-EMZL from normal eyes (area under ROC: 0.69–0.74).
Conclusions
Multiple tear cytokines were significantly dysregulated in OA-EMZL patients. These cytokines could potentially serve as diagnostic biomarkers and therapeutic targets in future.
Similar content being viewed by others
Introduction
Ocular adnexal lymphoma (OAL) is a malignant lymphoproliferative tumour predominantly affecting the conjunctiva, orbit and/or lacrimal gland [1]. Although OAL only accounts for ~1% of all lymphomas and ~8% of all extranodal lymphomas [2], it is the most common primary orbital malignancy in adults [3]. The incidence of OAL has been reported to be increasing, probably owing to the longer life expectancy worldwide over the years. Histologically, extranodal marginal zone B-cell lymphoma (EMZL) is the most frequent subtype, making up ~60% of all OALs [4]. Patients with primary OA-EMZL generally have a favourable clinical outcome, but they may face a continuous risk of distant relapse and transformation to more aggressive subtypes [5]. The exact pathogenesis of OA-EMZL has not been fully elucidated. Studies have shown that it related to multiple genetic and environmental factors from exposure to chronic antigenic stimuli and immune dysregulation to infectious agents[4, 6]. A better knowledge of the bioclinical changes in OA-EMZL tissue and the microenvironments would help uncover the pathophysiology and further provide the possibility for targeted treatment options.
Cytokines produced by cancer cells could sustain active but dysfunctional immune responses between tumour cells and the microenvironments [7], whereby maintaining the development and progression of tumours. Cytokine profiling of body fluids has offered an opportunity for the diagnosis, classification and revealing the aetiology of malignancies with minimal invasion [7]. As an ideal source of biomarkers for ocular disease, human tear fluid contains over 30 cytokines under physiological conditions [8]. By measuring tear film cytokine levels with enzyme-linked immunoassays, antibody array or high throughput assays, investigators have not only explored the inflammatory and immunologic mechanisms underlying ocular diseases, but also attempted to use them as biomarkers with regard to treatment response [9]. For example, increased levels of pro-inflammatory cytokines (e.g. IL-1β, IL-6 and tumour necrosis factor-alpha (TNF-α)) have been consistently documented in DED, and strongly correlated with dry-eye-related clinical parameters [10, 11]. Furthermore, longitudinal changes of such tear cytokines could serve as desirable markers for the effectiveness of treatment, for example topical steroids therapy [12] and intense pulsed light therapy [13]. With the emergence of a novel multiplex bead Luminex analysis technology, recent research on tear cytokine biosignatures has been extended to explore the aetiology of other diseases [14], like keratoconjunctivitis [15] and Sjögren’s syndrome [16].
OA-EMZLs frequently involve the tear secretory apparatus and sometimes the tear drainage system, such as the conjunctiva, the lacrimal gland and occasionally the eyelid. We thereby hypothesised that there might be changes in bio-molecular compositions, especially the cytokine profiles in OA-EMZL tears. Therefore, the purpose of the present study was to unveil the changes in tear cytokine profiles, and to investigate the clinical significance of such changes.
Materials and methods
Study population
Treatment-naïve, clinically diagnosed primary OA-EMZL patients were enroled at the Department of Orbital Diseases and Ocular Oncology, Zhongshan Ophthalmic Centre from December 11, 2018 to August 5, 2019. Tear samples of basal secretion were prospectively collected before treatment, but only those with a histopathologically verified OA-EMZL were finally included for cytokine profiling. Meanwhile, 14 eyes of 14 age- and sex-matched healthy control subjects were recruited from our department as well. The exclusion criteria for all participants were: (1) any concurrent ocular diseases other than OA-EMZL, (2) any history of ocular trauma or surgery, (3) contact lens wear within the previous 3 months, (4) female subjects during their menstruation, (6) use of topical corticosteroid within the previous 3 months. OA-EMZL patients with the following conditions were excluded: recurrent cases, any history of radiotherapy, chemotherapy or immune-modulating therapies. This study was approved by the Institutional Review Board of Zhongshan Ophthalmic Centre, Guangzhou, China. It was performed according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Systemic and ophthalmic examination
The medical history and demographic data were obtained through a brief interview. All subjects underwent comprehensive ophthalmic examination, including visual acuity, intraocular pressure, inspection and palpation of the eyelids, ocular motility assessment, anterior and posterior segment evaluation under slit lamp biomicroscope, and inspection for regional lymph node involvement. The tear breakup time (TBUT) was summarised as the mean of three measurements. The corneal and conjunctival staining pattern was graded from 0 (absent) to 5 (severe) according to the Oxford grading scale [17]. For each OA-EMZL patient, all conjunctival surfaces were assessed under slit lamp biomicroscope with eversion of the upper and lower eyelids. The extent of conjunctiva lymphoma was coded according to the quadrant involved: 0—not involved, 1—<1 quadrant, 2—1–2 quadrants, 3—2–3 quadrants, 4—≥3 quadrants. Contrast-enhanced orbital magnetic resonance imaging was conducted for all OA-EMZL subjects to determine the involvement of the lacrimal gland, extraocular muscles, or any other intra-orbital structures. Based on the anatomic sites involved, tumour was classified into four categories (T1–T4) by an experienced ocular oncologist (HSY) according to the American Joint Committee on Cancer (AJCC) Staging Manual (8th edition) [18]. The definition of primary tumour (T category) was as follows: T0, no evidence of lymphoma; T1, lymphoma involving the conjunctiva alone without eyelid or orbital involvement; T2, lymphoma with orbital involvement with or without conjunctival involvement; T3, lymphoma with preseptal eyelid involvement with or without orbital involvement and with or without conjunctival involvement; T4, orbital adnexal lymphoma and extraorbital lymphoma extending beyond the orbit to adjacent structures, such as bone, maxillofacial sinuses, and brain. A diagnostic, incisional biopsy of the lesion was performed for each OA-EMZL patient. Hematoxylin and eosin, and immunohistochemistry stained slides were reviewed by two senior pathologists to confirm lymphoma diagnosis and determine the histologic subtype.
Tear sample collection
Tear fluids were sampled during patients’ hospitalization for incisional biopsy (OA-EMZL group) or other ophthalmic surgeries (HC group). For patients bilaterally affected by OA-EMZL, only the eye scheduled for biopsy was enroled. Tears were collected before any ophthalmic examination to reduce reflex tear secretion. To minimize physiological diurnal variation of cytokines, tear was collected between 10:00 a.m. and 4:00 p.m. As previously described [19], a 20 μL glass capillary micropipette (Drummond, Broomall, PA, USA) was used to collect tear sample from the external canthus under slit lamp biomicroscope. After ~12 μL tear fluid was obtained from each eye, samples were immediately transferred to sterile Eppendorf tubes, sealed with Parafilm, and banked at −80 °C refrigerator until profiling assays performed.
Tear cytokine profiling
The Human Cytokine 27-Plex Panel (Luminex X-200, Invitrogen, Carlsbad, CA, USA) was used to measure the concentrations of tear cytokines according to the manufacturer’s instructions. In brief, diluted tear samples were incubated with capture beads, and then reacted with biotin-labelled antibodies. After incubation with streptavidin-phycoerythrin, resuspended samples were loaded to a 96-well plate for final analysis on the Luminex system. A standard curve for each cytokine was constructed based on the median fluorescence intensities (MFIs) and the corresponding concentrations (pg/mL). The concentration of every cytokine in each sample was extrapolated from the MFI and its respective standard curve. Two replicates were independently analysed for each sample to improve accuracy. In this study, the following 27 human cytokines were analysed: granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), interleukin-1 beta, IL-1 receptor antagonist (IL-1RA), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, interleukin-12 p70 (IL-12 P70), IL-13, IL-15, IL-17A, IFN-γ-induced protein 10 (IP-10), TNF-α, monocyte chemotactic protein-1, macrophage inflammatory protein-1-alpha, macrophage inflammatory protein-1-beta, regulated and normal T cell expressed and secreted, eosinophil chemotactic protein (Eotaxin), platelet-derived growth factor (PDGF) AB/BB, fibroblast growth factor-2 (FGF-2), and vascular endothelial growth factor (VEGF).
Statistical analysis
Descriptive statistics was summarised as mean ± SD, or median with range and interquartile range according to the statistical distribution of data. Differences in cytokine concentrations between groups were analysed using the nonparametric Mann–Whitney U test. Correlation between cytokine levels and clinical features in OA-EMZL group were performed using Spearman’s rank test. Statistical analyses were performed with the R software (version 3.5.1) and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). A P value < 0.05 was considered statistically significant unless otherwise indicated.
Results
A total of 35 eyes from 35 subjects were enroled in this study, with 21 (60.0%) OA-EMZL patients and 14 (40.0%) healthy subjects. The demographics and clinical features were summarised in Table 1. Overall, HC and OA-EMZL group had a comparable mean age (42.9 ± 12.9 vs. 45.6 ± 16.4 years, P = 0.612) and sex distribution (28.6% vs. 52.4% of male, P = 0.176) as expected from the age- and sex-matched design. No differences were found in smoking status, TBUT or Oxford grading of ocular surface staining between groups (all P > 0.05). In the OA-EMZL group, the distribution of AJCC 8th T stage was as follows: 8 (38.1%) of T1, 9 (42.9%) of T2 and 4 (19.0%) of T3. Involvements of conjunctiva and lacrimal gland were observed in 12 (57.1%) and 7 eyes (33.3%), respectively.
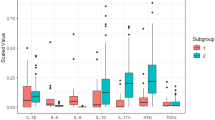
A total of 27 cytokines were analysed using the Luminex system. All experiments had measurable MFIs within the standard curve of each cytokine. Detailed comparisons of the 27 cytokine levels between HC and OA-EMZL tears were listed in Table S1. Of them, IL-1RA and IL-8 were significantly elevated in OA-EMZL tear samples as compared with those from healthy controls (P = 0.033 and 0.026, respectively). Three cytokines (FGF-2, IL-2 and IL-4, P < 0.05 for each) were significantly decreased and two (GM-CSF and IL-12 P70) were marginally significantly decreased in OA-EMZL tears (Table S1 and Fig. 1). Concentrations of the left twenty cytokines were comparable between two groups (P > 0.05 for each, Table S1). Of note, the ratio of anti-inflammatory IL-1RA to the pro-inflammatory IL-1β (IL-1RA/IL-1β) was significantly increased in the OA-EMZL group (P = 0.016), whereas the IL-10 to IL-6 ratio (IL-10/IL-6) was significantly decreased (P = 0.027, Fig. 1, Table S1). Unsupervised clustering of the dysregulated cytokines could separate all 35 subjects into two clusters based on the patterns of tear cytokine expression (Fig. 2). The cluster A contained 18 subjects with 14 OA-EMZL eyes (77.8%), while the cluster B contained 17 cases with ten controls (58.8%), showing a significantly different distribution (Fisher’s exact test P = 0.041). Notably, all T3 and 66.7% of T2 cases were classified into cluster A (Fig. 2).
HC healthy control, OA-EMZL extranodal marginal zone B-cell lymphoma (EMZL) of the ocular adnexa (OA), FGF-2 fibroblast growth factor-2, GM-CSF granulocyte macrophage colony-stimulating factor, IL-1RA IL-1 receptor antagonist. Differences between groups are analysed using the nonparametric Mann–Whitney U test. *P < 0.05.
The samples are represented in columns, and the cytokines represented in rows. Data are normalized as the z-score by each row. Relatively high and low concentration is indicated as red and blue colours, respectively, as shown in the scale bar. Upper covariate tracks show the group, T stage (T0–T3), LG involvement and conjunctiva involvement. HC healthy control, OA-EMZL extranodal marginal zone B-cell lymphoma (EMZL) of the ocular adnexa (OA), FGF-2 fibroblast growth factor-2, GM-CSF granulocyte macrophage colony-stimulating factor, IL-1RA IL-1 receptor antagonist, LG lacrimal gland.
For the 21 OA-EMZL patients, we performed Spearman’s rank correlation analyses between the clinical features and the levels of selected cytokines (i.e. FGF-2, GM-CSF, IL-1RA, IL-2, IL-4, IL-8 and IL-12 P70). As shown in Table 2, there were moderate negative associations between age and levels of FGF-2 (rs = −0.44, P = 0.043), GM-CSF (rs = −0.48, P = 0.026), IL-2 (rs = −0.44, P = 0.046), IL-12 P70 (rs = −0.44, P = 0.047) and IL-10/IL-6 ratio (rs = −0.47, P = 0.031). Tear concentrations of FGF-2, GM-CSF and IL-2 were all negatively associated with AJCC T stage (rs = −0.44, −0.49, −0.45, respectively; P = 0.043, 0.025, 0.042, respectively). Notably, lacrimal gland involvement was positively associated with IL-8 elevation (rs = 0.53, P = 0.012), and negatively associated with FGF-2 level (rs = −0.43, P = 0.049) and IL-10/IL-6 ratio (rs = −0.48, P = 0.026). We did not observe statistically significant correlations between any cytokine and gender or the extent of conjunctiva involvement (all P > 0.05). Similarly, no significant association between IL-1RA, IL-4 or IL-1RA/IL-1β and any studied clinical parameters was detected (Table 2).
We plotted receiver operating characteristic curves to assess the performance of aforementioned indices in OA-EMZL diagnosis. As demonstrated in Fig. 3, the areas under the curve (AUC) of each cytokine ranged from 0.69 to 0.72. The ratios of IL-1RA/IL-1β and IL-10/IL-6 achieved the greatest AUCs in discriminating OA-EMZL from HC (0.74 and 0.72, respectively, Fig. 3).
Discussion
The discovery of tear cytokine biomarkers could potentially substantiate their application in diagnosis, and in elucidating pathogenesis of ocular diseases non-invasively. In this study, we tested a panel of 27 human cytokines in OA-EMZL tears compared with healthy individuals. Significant differences were observed in five cytokines. Among them, the levels of IL-1RA and IL-8, as well as IL-1RA/IL-1β ratio, were elevated, while levels of FGF-2, IL-2, IL-4, and the ratio of IL-10/IL-6 were decreased in OA-EMZL tear fluid. The majority of classical pro-inflammatory (e.g. IL-1β, IL-6 and TNF-α) and pro-angiogenic factors (e.g. VEGF and PDGF) were comparable. Moreover, correlation analyses demonstrated associations between the levels of some cytokines and the tumour stage and the lacrimal gland involvement. These findings indicated that cytokine-associated inflammatory and immune mechanisms may be involved in OA-EMZL pathogenesis.
Given the critical roles of cytokine in mediating host response to cancer cells, dysregulation of cytokines in biofluids is reflective of the activity of cancer cells and the interaction with microenvironment. Studies on systemic lymphomas have documented the significance of serum cytokine as diagnostic or prognostic biomarkers [20, 21]. In ocular lymphomas, vitreous cytokine analysis has also been widely used in facilitating primary vitreoretinal lymphoma (PVRL) diagnosis, showing even higher sensitivity than vitreal cytology [22]. Herein, we present a novel analysis on cytokine in tear samples of OAL patients, added novel evidence on cytokines in the pathogenesis and as diagnostic biomarkers in lymphomas.
In our study, we found that IL-8 in OA-EMZL tear samples was significantly elevated and was positively associated with lacrimal gland involvement. Studied have documented that upregulation of IL-8 and its receptors are characterised in cancer cells and tumour-associated macrophages, exerting regulatory function in cancer microenvironment. Our finding of IL-8 elevation in OAL tear sample is well consistent with the existing findings in systemic lymphomas. For instance, in DLBCL and mantle cell lymphoma, abnormally elevated serum IL-8 has been reported and could serve as an independent prognostic predictor for unfavourable response to treatment, and for inferior event-free survival [23, 24]. In vitreoretinal lymphoma, IL-8 level was also elevated in vitreous fluid [25]. Comparable to the findings in those aggressive lymphomas, the elevation of serum IL-8 was documented in lower grade gastrointestinal mucosa-associated lymphoid tissue lymphoma [26], which was similar to our present results. Taken together, we assume that IL-8 might be associated with the development of a broad spectrum of B-cell lymphomas; both low- and high-grade lymphomas could develop and/or progress in the background of a high IL-8 microenvironment.
Our findings of the increased level of IL-1RA and decreased levels of several pro-inflammatory factors (IL-2 and IL-4) suggested an enhanced anti-inflammatory property in OAL microenvironment. IL-1RA naturally functions as an endogenous regulatory mechanism against IL-1β mediated inflammation and tissue damage. The balance between anti-inflammatory IL-1RA and pro-inflammatory IL-1β potentially could affect carcinogenesis and tumour progression [27]. IL-1RA upregulation and the imbalance between IL-1RA and IL-1β have been documented in several lymphoma subtypes. For example, serum IL-1RA increase was associated with systemic DLBCL and FL [24]. In PVRL, increased IL-1RA level was recently detected in vitreous specimens as well [28]. In this study, we demonstrated an elevated level of IL-1RA in the OA-EMZL tear fluid, which was in agreement with existing evidence. Considering the increased ratio of IL-1RA/IL-1β, we postulated that the anti-inflammatory reactions might overweigh the pro-inflammatory ones in OA-EMZL. As the increased anti-inflammatory property is often indicative of the inhibition of macrophage function, so our finding might indicate that OA-EMZL tumour cells attempt to establish a homeostasis through dampened local inflammatory reaction.
We surprisingly found that the classical pro-angiogenic factors, including FGF-2, VEGF and PDGF, were either decreased or unchanged in OA-EMZL tears. Such cytokines and their receptors are widely expressed in solid tumour tissues and are often related to invasive tumour growth and distant metastasis. For example, expression of FGF-2 was found to be significantly elevated in sera and tissue specimens of Hodgkin’s and non-Hodgkin’s lymphomas. The overall low levels of pro-angiogenic factors in tear samples might be reflective of the low-grade malignancy and favourable prognosis of OA-EMZL.
Despite the pronounced differential expression in OA-EMZL tears, most cytokines were not significantly correlated with the AJCC T stage except for FGF-2, GM-CSF and IL-2. With greater tumour stage, there were lower concentrations of those three cytokines in tears, indicating the synthesis and/or secretion are inhibited following tumour growth. As for the anatomic site involvement and cytokine changes, the correlation analysis implied that the severity of conjunctiva involvement might not contribute to the alteration of tear cytokines. Of note, invasion of lacrimal gland might be responsible for the decrease of FGF-2 and elevation of IL-8. The exact biological mechanism under these cytokine production remains to be further investigated.
Translationally, our study provides the preliminary evidence that the tear cytokine profiling can be used as a non-invasive diagnostic approach for OA-EMZL. It would be interesting to further investigate its performance in predicting prognostic outcomes, such as relapse and survival in longitudinal studies. The therapeutic implications of our findings are also worthy of investigation. For instance, as IL-8 was significantly elevated in OA-EMZL tears, local therapy targeting IL-8 or its downstream signalling pathway might a novel and unexploited option. Likewise, as several cytokines were downregulated in OA-EMZL (e.g. IL-2 and IL-4), it is also interesting to assess the benefit of adding cytokine supplementary to current standard therapy.
Strength of this study is the use of the high throughput and reliable Luminex technique in tear cytokine profiling. It provided detailed information of cytokine patterns simultaneously through analysing minimal volumes of specimen. However, there were several limitations to be addressed as well. First, all OALs in our study are of low-grade EMZL subtype. Further research including more aggressive subtypes is required to confirm our results before generalization. Second, owing to the small sample size (14 HC eyes and 21 OA-EMZL eyes), there was inadequate statistical power allowing subgroup analyses by tumour stage. The insignificant correlation between tumour stage and increased cytokines (IL-1RA and IL-8) might probably attribute to the few participants in the T3–T4 group. Third, molecular genetic analysis was not conducted for all OA-EMZL patients, thus we could not correlate the cytokine levels with common translocations and genetic changes.
In conclusion, our research revealed unique patterns of tear cytokine from OA-EMZL patients. The concentration of several cytokines was associated with the tumour stage. This study added clues to the pathogenesis, and might pave the way for establishing novel therapeutic targets for OA-EMZL. Longitudinal studies are required to validate the performance of tear cytokine testing in predicting prognosis, and to evaluate the effectiveness and efficacy of targeted therapy in OA-EMZL treatment.
Supplemental information is available on Eye’s website.
Summary
What was known before
-
Analysing tear cytokine biosignatures has increasingly helped uncover the pathogenesis of many ocular diseases.
What this study adds
-
Multiplex bead array assay revealed that the concentrations of multiple pro- and anti-inflammatory cytokines in tear fluid were significantly changed in eyes with ocular adnexal marginal zone B-cell lymphoma.
References
Coupland SE, White VA, Rootman J, Damato B, Finger PT. A TNM-based clinical staging system of ocular adnexal lymphomas. Arch Pathol Lab Med. 2009;133:1262–7.
Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–60.
Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111:997–1008.
Sassone M, Ponzoni M, Ferreri AJ. Ocular adnexal marginal zone lymphoma: clinical presentation, pathogenesis, diagnosis, prognosis, and treatment. Best Pr Res Clin Haematol. 2017;30:118–30.
Desai A, Joag MG, Lekakis L, Chapman JR, Vega F, Tibshirani R, et al. Long-term course of patients with primary ocular adnexal MALT lymphoma: a large single-institution cohort study. Blood. 2017;129:324–32.
Ferreri AJ, Dolcetti R, Magnino S, Doglioni C, Ponzoni M. Chlamydial infection: the link with ocular adnexal lymphomas. Nat Rev Clin Oncol. 2009;6:658–69.
Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:218–28.
Carreno E, Enriquez-de-Salamanca A, Teson M, Garcia-Vazquez C, Stern ME, Whitcup SM, et al. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010;88:250–8.
Zhou L, Beuerman RW. The power of tears: how tear proteomics research could revolutionize the clinic. Expert Rev Proteom. 2017;14:189–91.
Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205.
Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59:DES192–DES199.
Lee H, Chung B, Kim KS, Seo KY, Choi BJ, Kim TI. Effects of topical loteprednol etabonate on tear cytokines and clinical outcomes in moderate and severe meibomian gland dysfunction: randomized clinical trial. Am J Ophthalmol. 2014;158:1172–83.
Liu R, Rong B, Tu P, Tang Y, Song W, Toyos R, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90.
Hagan S, Tomlinson A. Tear fluid biomarker profiling: a review of multiplex bead analysis. Ocul Surf. 2013;11:219–35.
Sun YC, Tang YH, Liou HM, Chen WL, Hu FR. Tear cytokine profiling in patients with superior limbic keratoconjunctivitis who underwent medical treatment or in conjunction with surgical management. Br J Ophthalmol. 2019;104:735–40.
Chen X, Aqrawi LA, Utheim TP, Tashbayev B, Utheim OA, Reppe S, et al. Elevated cytokine levels in tears and saliva of patients with primary Sjogren’s syndrome correlate with clinical ocular and oral manifestations. Sci Rep. 2019;9:7319.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50.
Heegaard S, Chevez-Barrios P, White VA, Coupland SE, Finger PT. Ophthalmic sites: ocular adnexal lymphoma. In: Amin MB, editor. AJCC cancer staging manual. 8th ed. New York: Springer, 2017. p. 857–62.
Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernandez I, Carreno E, Garcia-Vazquez C, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73.
Mir MA, Maurer MJ, Ziesmer SC, Slager SL, Habermann T, Macon WR, et al. Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood. 2015;125:992–8.
Gupta M, Stenson M, O’Byrne M, Maurer MJ, Habermann T, Cerhan JR, et al. Comprehensive serum cytokine analysis identifies IL-1RA and soluble IL-2Ralpha as predictors of event-free survival in T-cell lymphoma. Ann Oncol. 2016;27:165–72.
Frenkel S, Pe’er J, Kaufman R, Maly B, Habot-Wilner Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020;98:e668–e673.
Sonbol MB, Maurer MJ, Stenson MJ, Allmer C, LaPlant BR, Weiner GJ, et al. Elevated soluble IL-2Ralpha, IL-8, and MIP-1beta levels are associated with inferior outcome and are independent of MIPI score in patients with mantle cell lymphoma. Am J Hematol. 2014;89:E223–227.
Charbonneau B, Maurer MJ, Ansell SM, Slager SL, Fredericksen ZS, Ziesmer SC, et al. Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: a clinic-based case-control study. Cytokine. 2012;60:882–9.
Usui Y, Wakabayashi Y, Okunuki Y, Kimura K, Tajima K, Matsuda R, et al. Immune mediators in vitreous fluids from patients with vitreoretinal B-cell lymphoma. Invest Ophthalmol Vis Sci. 2012;53:5395–402.
Miyata-Takata T, Takata K, Toji T, Goto N, Kasahara S, Takahashi T, et al. Elevation of serum interleukins 8, 4, and 1beta levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci Rep. 2015;5:18434.
Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281:57–61.
de Hoog J, Dik WA, Lu L, Heezen KC, Ten Berge JC, Swagemakers SMA, et al. Combined cellular and soluble mediator analysis for improved diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2019;97:626–32.
Acknowledgements
We thank all patients involved in this study. This study was supported by the National Natural Science Foundation of China (81670887, 81870689, 81700820), the Natural Science Foundation of Guangdong Province, China (2017A030313613, 2016A030310230) and the Pearl River Nova Programme of Guangzhou (201806010167).
Funding
This study was supported by the National Natural Science Foundation of China (81670887, 81870689, 81700820, 81600751), the Natural Science Foundation of Guangdong Province, China (2017A030313613, 2016A030310230) and the Pearl River Nova Program of Guangzhou (201806010167).
Author information
Authors and Affiliations
Contributions
Conceptualization: WX, XC and HY. Data curation: WX and YM. Formal analysis: WX and XJ. Investigation: JC, HY and XJ. Methodology: XJ and SY. Project administration: HY. Supervision: YM and HY. Validation: XJ. Visualization: WX. Writing—original draft: WX and XC. Writing—review and editing: SY, YM and HY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent for publication
Written patient consent obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiao, W., Chen, J., Ye, H. et al. Tear cytokine profiles in patients with extranodal marginal zone B-cell lymphoma of the ocular adnexa. Eye 36, 1396–1402 (2022). https://doi.org/10.1038/s41433-021-01650-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01650-7