Abstract
Objective
To analyse the central corneal thickness, endothelial cell density and morphology in patients with diabetes mellitus (DM).
Methods
We analysed corneal endothelium, i.e. central corneal thickness (CCT), endothelial cell density (ECD), coefficient of variation in cell size (CV), and hexagonality (Hex) with specular microscopy in patients with type 2 DM and compared with age-matched controls. The influence of diabetic retinopathy (DR) severity, duration of DM, and level of glycosylated haemoglobin (HbA1c) was also analysed.
Results
The study cohort included 592 eyes of 592 diabetic patients and 596 eyes of 596 control subjects. A significant difference was found in CCT (522.1 ± 36.6 μm in DM, 514.9 ± 37.1 μm in controls; P = 0.001), ECD (2484.5 ± 299.5 cells/mm2 in DM, 2555.9 ± 258.2 cells/mm2 in controls; P = 0.017), CV (40.3 ± 6.1 in DM, 37.2 ± 6.1 in controls; P < 0.001) and Hex (39.9 ± 5.2 in DM, 44.6 ± 6.0 in controls; P < 0.001). The longer duration of DM ( > 10 years) and poor glycaemic control (HbA1c > 7.5%) were associated with similar results. A significantly reduced ECD (P < 0.001) and Hex (P = 0.001) and higher CV (P = 0.007) and CCT (P = 0.01) was noted when assessed against various stages of DR. Multivariate analysis showed that increasing age was significantly associated with lower ECD (P < 0.001), Hex (P < 0.001), and CCT (P = 0.004); and a higher CV (P < 0.001).
Conclusions
DM has deleterious effects on corneal endothelium and thickness. The presence of DR may further warrant a thorough corneal evaluation, especially when planning intraocular surgery.
Similar content being viewed by others
Diabetes mellitus is a major health hazard reaching epidemic proportions worldwide [1]. With an estimated 134 million people affected by this systemic disease, India is expected to top the list by the year 2045 [1, 2]. Being a metabolic disease, DM affects every part of the body and the eye is not an exception [3, 4]. It is a major cause of visual loss in the working-age population worldwide [4]. Even though retinopathy is the most common sequel and most widely studied association of DM, other structures of the eye are not immune and get affected in various stages of the disease [4,5,6].
The most recognised corneal complication in DM (both type I and type II) is keratopathy [5] resulting from impaired epithelial basement membrane (BM), epithelial wound healing, epithelial–stromal interactions, endothelial function, and corneal nerve functions [7]. The resultant morphological and functional alterations impart an increased susceptibility of the cornea to pathologies like recurrent corneal erosions, superficial keratitis, punctate epithelial keratopathy, persistent epithelial defects leading to recurrent ulceration, impaired corneal sensitivity, and slowed healing capacity following trauma or surgical insult [6,7,8,9,10,11,12,13]. These complications, especially after cataract or vitreoretinal surgeries, are a major concern due to possible corneal decompensation.
Considering the indispensable role of corneal endothelium in maintaining corneal clarity, several researchers have investigated the possible alterations that take place in endothelium in patients with DM. A damaged endothelium results in corneal oedema, and increased central corneal thickness (CCT), among other causative factors. This increase has been documented in patients with DM as compared with healthy controls in a large number of studies [14,15,16,17,18,19]. Some studies have shown a significant correlation of CCT to the duration of DM [19], whereas a study conducted by Choo et al showed no correlation of the duration of DM, glycosylated haemoglobin (HBA1c) level, and severity of diabetic retinopathy (DR) with any of the corneal morphological parameters [18]. The other parameters like coefficient of variation (CV), endothelial cell density (ECD), and hexagonality of cells (Hex) have also been a focus of interest in many studies, but the results have been inconsistent. A study by Schultz et al showed a higher CV and a reduced Hex in patients with type I and II DM, with no difference in ECD as compared with non-diabetics [20]. Similar results were published by Larsson et al. [21] and Hugod et al. [22] and it was postulated that these changes resembled those of aging cornea and could not be differentiated from the older population of patients with type 2 DM. A study by Keoleian et al. showed no significant changes observed in CT and ECD, although a higher CV and a reduced Hex were noted similar to other studies [23]. The variability of these morphological changes is evident from the recent study by Paulsen et al who showed a significant increase in CCT in patients with DM, with no significant changes noted in ECD CV or Hex [16]. As the duration of DM was not taken into account in this study, it is extremely difficult to extrapolate these results.
Interestingly, very few studies have analysed the association of the severity of DR [18, 21] and the duration of DM [19] with the altered corneal endothelial parameters. A recent study by El-Agami et al found no significant changes in these parameters with the severity of DR, and no correlation with duration of DM, HbA1c, and DR severity [24], whereas a study by Elsobky et al. showed that the corneal parameters were significantly correlated with the duration and DR severity [25]. They divided the DR into no DR, non-proliferative DR and proliferative DR. As of date, there is no conclusive evidence in the literature about the association of DR severity on these parameters. It is possible that the severity of DR may not directly impact corneal health. This may signify an effect of factors like duration, severity of DM and, age, etc. which has been proven in the past. However, assessment of the corneal health takes precedence when a surgical intervention like cataract surgery is planned. The presence of DR and its severity may warrant a thorough pre-operative corneal assessment, especially in the Indian population with a large number of cataract surgeries being performed every year.
Methods
Study population
The study cohort consisted of 1188 corneas of 1188 subjects in this prospective, observational, institutional review board (Military Hospital, Jammu, India) approved study. These were further divided into a group with type 2 diabetes mellitus (N = 592), and healthy controls (N = 596). All the subjects were recruited from the clinic at Military Hospital, Jammu, India, and signed written informed consent prior to enrolment. This study adhered to the tenets of the Declaration of Helsinki.
The patients in the DM group were identified based on the history, fasting blood sugar (≥126 mg/dL) and post-prandial (≥200 mg/dL); and glycosylated haemoglobin (HbA1c ≥ 6.5%) [26]. The healthy control subjects were recruited randomly among the patients who presented to our hospital. The data about the duration of DM and the presence and/or severity of diabetic retinopathy (DR) was also acquired in the DM group as detailed later.
Major exclusion criteria
Conditions affecting or altering the health of corneal endothelium like corneal endothelial dystrophies, prior ophthalmic surgeries, severe ocular trauma, prolonged contact lens use, raised intraocular pressure, high myopia, pterygium, previous retinal photocoagulation or intravitreal injection, present or past uveitis, known tear-interfering systemic drugs (such as anti-histaminic or hormone replacement therapy), a systemic illness known to impair tear function such as rheumatoid arthritis and systemic lupus erythematosus were excluded.
Grading protocol for corneal parameters
Following a comprehensive ophthalmic evaluation including visual acuity assessment by Snellen’s chart, anterior segment examination by slit-lamp biomicroscopy, intraocular pressure measurements by applanation tonometer, dilated fundus evaluation by indirect ophthalmoscopy, the study eyes underwent corneal endothelial evaluation by non-contact specular microscopy (EM 3000 Tomey Nishi-Ku, Nagoya, Aichi, Japan).
The corneal endothelial parameters i.e. central corneal thickness (CCT; µm), endothelial cell density (ECD; cells/mm2), coefficient of variation in cell size (CV; %), and hexagonality (Hex; %) were recorded using the specular microscope with an auto-tracking system. Software integrated into the system, ImageNet, computes the endothelial cell layer with high precision. The right eyes for all patients were chosen for analysis purposes. Three corneal images were captured by the masked technician at the time of image acquisition, and an average was recorded for subsequent analysis.
Grading of diabetic retinopathy
The DM group was assessed for the duration of DM as well as glycaemic control by analysing the HbA1c values at presentation to the clinic. They were also further classified into various grades of diabetic retinopathy based on the ICDR grading system as no apparent retinopathy, mild, moderate, severe non-proliferative DR (NPDR), and proliferative DR (PDR) for sub-group analysis [27]. All the patients had treatment naïve DR. The corneal analysis was done prior to any treatment given for DR.
Sample size calculation
The sample size was calculated after considering an effect size of clinical interest (d), that is difference in means of endothelial ECD as 95 cells/mm2; combined standard deviation (SD) as 296 (after pilot survey); Z alpha (Za) as 1.96 (corresponding to type I error of 5%, i.e. 0.05) and Z beta (Zb) as 0.84 (corresponding to power of 80%). The number of eyes in each group was calculated as follows: by considering the combined SD of CV for DM and Non-DM patients, we calculated the sample size by using following formula
n = sample size (for BWT comparison);
Zα = Standard normal variate for α = 0.05 (95% CI) = 1.96;
Z1 − β = Standard normal variate for 1 − β = 0.80 (80%) = 0.84;
Combined SD = 9.72;
Effective size = d = 1.75;
Minimum required sample size per group was 484.
Statistical analysis
Data analysis was done by using SPSS (Statistical Package for Social Sciences) version V.25.0. Quantitative data variables were expressed in mean ± standard deviation (for normally distributed data) and median with inter-quartile range (IQR) for the rest. Mann–Whitney U test was used to compare the difference of corneal parameters in both groups. Kruskal–Wallis test was used to assess the difference of corneal parameters between different groups of diabetic retinopathy severity; post-hoc analysis was performed to further investigate the difference between the specific group pairs. Multivariate regression analysis was performed to analyse the effect of independent variables like age, DM duration, HBA1c and DR severity, on the study parameters. Spearman’s correlation analysis was performed to evaluate the relationship between corneal parameters and the duration of diabetes, HbA1c level, and grades of DR. Probability value, P < 0.05 was considered statistically significant.
Results
The study cohort comprised of 1188 eyes of 1188 subjects. The groups were matched for age and gender. The mean age of subjects in the DM and the control groups was 62.17 ± 9.49 years (range, 46–90 years) and 63.03 ± 11.04 years (range, 44–88 years), respectively (P = 0.152). As mentioned before, all eyes were phakic. The distribution of subjects according to the duration, HbA1c level, and DR status is shown in Table 1.
On comparing the corneal parameters between the corneas of DM and healthy subjects (Table 2), a significantly increased CCT (522.1 ± 36.6 μm in DM, 514.9 ± 37.1 μm in controls; P = 0.001) and CV (40.3 ± 6.1 in DM, 37.2 ± 6.1 in controls; P < 0.001); and a significantly lower ECD (2484.5 ± 299.5 cells/mm2 in DM, 2555.9 ± 258.2 cells/mm2 in controls; P = 0.017) and Hex (39.9 ± 5.2 in DM, 44.6 ± 6.0 in controls; P < 0.001) were noted. Similar significant results were obtained with age-wise stratification of the subjects, except the ECD in the 40–49 years age group and the CCT in 60–69 years and >70 years age groups which were not significantly different between the two groups. Within the DM group (Table 3), longer duration of DM ( > 10 years), and higher HbA1c (>7.5%) levels were also associated with significantly higher CCT and CV; and lower ECD and Hex as compared to those with shorter duration and lower HbA1c value.
When compared between no DR and various grades of DR with post-hoc analysis to compare between each group, similar results were obtained (Table 4) with statistically reduced ECD (P < 0.001) and Hex (P = 0.001); and higher CV (P = 0.007) and CCT (P = 0.01).
Spearman correlation between duration, HbA1C and DR status, and the corneal parameters showed a significant negative correlation for ECD and Hex, and a significant positive correlation for CCT and CV, respectively (Table 5).
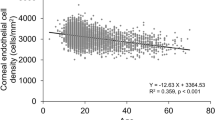
Multivariate regression analysis showed that increasing age was significantly associated with lower ECD (P < 0.001), Hex (P < 0.001), and CCT (P = 0.004); and a higher CV (P < 0.001). Also, increasing HbA1c was associated with significantly lower ECD (P < 0.001) and a higher CV (P = 0.002). DM duration and DR severity did not show any significant association with the study parameters.
Discussion
The results of this study showed that diabetic patients had significantly thicker corneas as well as an altered morphology i.e. increased CV of the cell area (polymegathism), and decreased cell density and hexagonality (pleomorphism) when compared with healthy controls. We also found similar results in both groups after stratification for age. In fact, increasing age was seen to be independently associated with such changes in multivariate regression analysis and poor glycaemic control, as indicated by higher HbA1c, was associated with a lower ECD and a higher CV. In the diabetic group, the duration of >10 years and HbA1c of >7.5% was associated with thicker corneas as well as increased polymegathism and pleomorphism. Our results were comparable to the study conducted by Lee et al. in 2006 who, in addition, noted a significant correlation of the duration of DM of >10 years with increased CCT and increased CV of cell size [19]. The resultant cornea with altered morphology and functionality is known to be more susceptible to pathologies like recurrent corneal erosions, and impaired corneal sensitivity following trauma or surgical insult leading to recurrent ulceration with impaired healing [6,7,8,9,10,11,12,13].
Most of these results have been published in the literature in past but without any uniform consistency. Although Choo et al. in 2010 showed similar results to our study in terms of thickness and endothelial morphology between diabetic and healthy eyes, their study did not show any correlation to the duration of DM, HBA1c level, and severity of DR [18]. A study by Schultz et al in 1984 showed a higher CV and a reduced Hex in patients with type I and II DM, with no difference in ECD as compared with non-diabetics [20]. Similar results were published by Larsson et al. [21] in 1996 and Hugod et al. [22] in 2011 and it was postulated that these changes resembled those of aging cornea and could not be differentiated from the older population of patients with type 2 DM. This was indeed the case in our study when we analysed the corneal morphology in the DM group and healthy eyes individually and found that these changes showed significant results with increasing age [28, 29]. The variability of these morphological changes is further evident from the recent study by Paulsen et al. in 2014 who showed a significant increase in CCT, with no significant changes noted in ECD, CV or Hex in patients with DM [16], and El-Agamy et al. in 2017 who showed a decreased ECD and increased CV in diabetic eyes, but no significant changes in Hex and CCT [24].
Certain changes in diabetic corneas have been noted which are thought to cause changes noted above. Tight apical junctions on the endothelial cells function as physical barriers; and the ion pumps in the endothelial cells are mainly responsible for the movement of water from the corneal stroma into the anterior chamber [30,31,32]. Diabetic cornea with high glucose can lead to increased sorbitol inside the cells due to increased activity of aldose reductase, which acts as an osmotic agent with subsequent cellular swelling. A reduced Na+/K+ ATPase activity in the endothelial cells in these eyes also results in altered permeability and ultimate destruction of these cells. Furthermore, defective endothelial pump function due to decreased ATP production has also been noted in diabetic corneas [18, 33, 34].
Increased duration (>10 years) of DM and poor glycaemic control (HbA1c > 7.5%) showed significantly reduced ECD and Hex and higher CCT and CV in this study. Also, univariate analysis in the DM group showed a statistically significant association of HbA1c with ECD (P = 0.002), CV (P = 0.002), and CCT (P = 0.030); whereas the duration of DM showed a statistically significant association with ECD (P = 0.03) and CV (P = 0.04). Hex was not associated with either the duration or the HbA1c. The study by Storr-paulsen et al. in 2013 showed that a higher HbA1c was associated with significantly lower endothelial cell counts [16]. Conversely, Choo et al. in 2010 did not find significant changes taking place in the cornea with increased duration or Hb1Ac [18], and Lee et al. in 2006 showed a higher CCT and CV in patients with a duration of DM > 10 years [19]. In fact, a significant correlation of HbA1c to CCT has been evaluated in studies by Lee et al. in 2006 and Su et al. in 2008 [35]. We believe that a larger cohort tested in our study exposed this association of corneal changes with longer duration and poor glycaemic control.
Our study also established for the first time that as the DR grade worsens, the CCT and the CV increase, whereas the ECD and Hex decrease significantly. Very few studies in the past have analysed these changes with DR severity and found no significant difference between the eyes with DR and with no DR [12, 18, 24]. Shenoy et al. showed that ECD was significantly lower in eyes with higher DR grades [36]. Recently Durukan in 2019 confirmed the association of reduced ECD and Hex in eyes with higher grades of DR [11]. Correlation analysis between the duration, HbA1c and DR status and the corneal parameters showed a significantly negative correlation for ECD and Hex (pleomorphism), and a significant positive correlation for CV (polymegathism) and CCT, respectively.
As India harbours a large population of patients with diabetes and DR especially in rural areas [37], it becomes imperative that any patient with uncontrolled DM or any level of DR may be subjected to stringent corneal evaluation to analyse the endothelial health. Any surgical intervention would thus result in better visual outcomes and a lesser rate of corneal complications, as these patients with DM are already at a higher risk of losing vision due to corneal decompensation [38]. It may transpire to be an important biomarker for endothelial dysfunction in patients with any level of DR for a careful follow-up and management of these patients.
Our study has many strengths. The study cohort is large with strict exclusion criteria to exclude any factors impacting corneal endothelial parameters and CCT. This study also showed a uniform impact of diabetes on increasing thickness and altered corneal morphology resulting in polymegathism and pleomorphism. Also, this is the first study showing conclusive evidence of the changes seen in corneal parameters with various grades of DR. This study is also not without limitations. All the limitations of a cross-sectional design apply to our study as well. The study cohort was limited to Asian ethnicity of Indian origin, and therefore, generalisation of these results in other populations is cautioned by the authors. Also, genetic studies in diabetics can reveal additional phenotypes altering corneal morphology ad functionality. Longitudinal studies with a long-term follow up and in different ethnicities are required to analyse the corneal changes in these eyes.
Conclusion
Long-term poor glycaemic control in patients with diabetes may be associated with corneal morphological and functional changes, which can be detrimental to the ultimate health of cornea over long-term, in health, injuries, and surgeries like cataract extraction. These may be exaggerated with associated retinopathy and must be monitored closely.
Summary
What was known before
-
Inconsistent association between various corneal endothelial parameters and Diabetes Extreme paucity of studies relating the severity of diabetic retinopathy with corneal changes.
What this study adds
-
Increasing severity of diabetic retinopathy is associated with corneal endothelial changes.
References
India State-Level Disease Burden Initiative Diabetes Collaborators. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6:e1352–62.
Sanchez Thorin JC. The epidemiology of diabetes mellitus and diabetic retinopathy. Int Ophthalmol Clin. 1998;38:11–8.
Jeganathan VSE, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31:1905–12.
Rowe NG, Mitchell PG, Cumming RG, Wans JJ. Diabetes, fasting blood glucose and age related cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2000;7:103–14.
Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012;8:294–302.
Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt. 1998;65:224–30.
Saghizadeh M, Kramerov A, Yu F, Castro M, Ljubimov A. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–80.
McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–16.
Roszkowska AM, Tringali CG, Colosi P, Squeri CA, Ferreri G. Corneal endothelium evaluation in type I and type II diabetes mellitus. Ophthalmologica. 1999;213:258–61.
Inoue K, Kato S, Inoue Y, Amano S, Oshika T. The corneal endothelium and thickness in type II diabetes mellitus. Jpn J Ophthalmol. 2002;46:65–9.
Shenoy R, Khandekar R, Bialasiewicz A, Al Muniri A. Corneal endothelium in patients with diabetes mellitus: a historical cohort study. Eur J Ophthalmol. 2009;19:369–75.
Sudhir RR, Raman R, Sharma T. Changes in the corneal endothelial cell density and morphology in patients with type 2 diabetes mellitus: a population-based study, Sankara Nethralaya Diabetic Retinopathy and Molecular Genetics Study (SN-DREAMS, Report 23). Cornea. 2012;31:1119–22.
Thomas N, Jeyaraman K, Asha HS, Velevan J. A Practical Guide to Diabetes Mellitus. New Delhi, India: Jaypee Brothers Medical Publishers (2012).
Rosenberg M, Tervo T, Immonen I, Muller L, Gronhagen-Riska C, Vesaluoma M. Corneal Structure and Sensitivity in Type 1Diabetes Mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–21.
Claramonte PJ, Ruiz-Moreno JM, Sánchez-Pérez SI, León M, Griñó C, Cerviño VD, et al. Variation of central corneal thickness in diabetic patients as detected by ultrasonic pachymetry. Arch Soc Esp Oftalmol. 2006;81:523–6.
Storr-Paulsen A, Singh A, Jeppesen H, Norregaard J, Thulesen J. Corneal endothelial morphology and central thickness in patients with type II diabetes mellitus. Acta Ophthalmol. 2013;92:158–60.
Busted N, Olsen T, Schmitz O. Clinical observations on corneal thickness and the corneal endothelium in diabetes mellitus. Br J Ophthalmol. 1981;65:687–90.
Choo M, Prakash K, Samsudin A, Soong T, Ramli N, Kadir A. Corneal changes in type II diabetes mellitus in Malaysia. Int J Ophthalmol. 2010;3:234–6.
Lee J, Oum B, Choi H, Lee J, Cho B. Differences in corneal thickness and corneal endothelium related to duration in diabetes. Eye. 2006;20:315–8.
Schultz RO, Matsuda M, Edelhauser HF, Yee RW, Schultz KJ. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984;98:401–10.
Larsson LI, Bourne WM, Pach JM, Brubaker RF. Structure and function of the corneal endothelium in diabetes mellitus type I and type II. Arch Ophthalmol. 1996;114:9–14.
Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J. Corneal endothelial cell changes associated with cataract surgery in patients with type II diabetes mellitus. Cornea. 2011;30:749–53.
Keoleian GM, Pach JM, Hodge DO, Trocme SD, Bourne WM. Structural and functional studies of the corneal endothelium in diabetes mellitus. Am J Ophthalmol. 1992;113:64–70.
El-Agamy Amira, Alsubaie Shams. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 2017;11:481–6.
Elsobky HMK, Farid FMW, El-Sayed EEM. Corneal endothelial and central corneal thickness changes in patients with type II diabetes mellitus. Menoufia Med J. 2019;31:1317–23.
Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. American Diabetes Association. Diabetes Care 2019; 42(Supplement 1): S13–28. https://doi.org/10.2337/dc19-S002.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh ECD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular oedema disease severity scales. Ophthalmology. 2003;110:1677–82.
Jorge J, Queirós A, Peixoto-de-Matos SC, Ferrer-Blasco T, González-Méijome JM. Age-related changes of corneal endothelium in normal eyes with a non-contact specular microscope. J Emmetropia. 2010;1:132–9.
Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95:16–23.
Bierhaus A, Hofmann M, Zeigler R, Nawroth P. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus I. The AGE concept. Cardivasc Res. 1998;37:586–600.
Yanagiya N, Akiba J, Kado M, Yoshida A, Kono T, Iwamoto J. Transient corneal oedema induced by nitric oxide synthase inhibition. Nitric Oxide. 1997;1:397–403.
Wigham C, Guggenheim J, Hodson S. Sodium movement into and out of corneal endothelium. Pflug Arch. 1994;428:577–82.
Browning DJ. Diabetic retinopathy: evidence-based management. New York, NY: Springer (2010).
Tripathy BB, Chandalia HB, Das AK, Rao PV. Textbook of Diabetes Mellitus. New Delhi, India: Jaypee Brothers Medical Publishers (2012).
Su DH, Wong TY, Wong WL, Saw SM, Tan DT, Shen SY, et al. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008;115:964–8.
Durukan I. Corneal endothelial changes in type 2 diabetes mellitus relative to diabetic retinopathy. Clin Exp Optom. 2020;103:474–8.
Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005.
Sharma N, Singhal D, Nair SP, Sahay P, Sreeshankar SS, Maharana PK. Corneal oedema after phacoemulsification. Indian J Ophthalmol. 2017;65:1381–9.
Author information
Authors and Affiliations
Contributions
AJ was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, updating reference lists, and creating ‘Summary of findings’ tables. AV was responsible for designing the review protocol and screening potentially eligible studies. He also contributed to design the paper and conduct in depth analysis. ARA was instrumental in conducting and designing the analysis and tables and formulate the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jha, A., Verma, A. & Alagorie, A.R. Association of severity of diabetic retinopathy with corneal endothelial and thickness changes in patients with diabetes mellitus. Eye 36, 1202–1208 (2022). https://doi.org/10.1038/s41433-021-01606-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01606-x
This article is cited by
-
Corneal endothelial morphology changes in patients with proliferative diabetic retinopathy
International Journal of Diabetes in Developing Countries (2024)
-
Corneal topographic indices of scheimpflug camera in type 2 diabetic and non-diabetic elderly populations
BMC Ophthalmology (2023)
-
Effects of chewing tobacco on corneal endothelium in patients with diabetes mellitus
Eye (2023)
-
Diabetic retinopathy and corneal endothelial parameters: an analytical cross-sectional study
BMC Ophthalmology (2022)