Abstract
Varied options are available for the implantation of secondary intraocular lens implants in the absence of zonular or capsular support. Loss of the capsule can occur in the context of complicated cataract surgery, trauma or inherited conditions such as Marfan syndrome or pseudoexfoliation. Approaches to overcome this include optical measures such as the use of spectacles or contact lenses, and surgical therapy incorporating the use of anterior chamber, iris-fixated or scleral-fixated lenses. Surgical techniques to implant scleral-fixated lenses have undergone various modifications, since the first publication of sutured intrascleral fixation described in the 1980s. However, despite the advances in surgical techniques, studies are limited either by their retrospective nature, small sample size and most importantly small duration of follow-up. This comprehensive review aims to amalgamate the evolution of various surgical techniques with regards to intrascleral lens fixation and suggests areas for future development.
摘要
经巩膜缝线固定的眼内人工晶状体植入术——手术技术的演变与未来发展
摘要
在缺乏晶状体悬韧带或囊膜支撑的情况下, 二期人工晶状体植入有多种选择。在复杂性白内障手术、外伤或遗传性疾病 (如马凡氏综合征或假性脱落) 的白内障手术中, 可能会出现晶状体囊膜缺失的情况。而光学治疗措施 (佩戴框架眼镜或隐形眼镜) 以及手术方法 (结合使用前房、虹膜固定或巩膜固定的人工晶状体) 可以解决问题。自20世纪80年代首次发表关于巩膜内固定术的文章以来, 植入经巩膜缝线固定人工晶体的外科技术经历了各种各样的改进。然而, 尽管外科手术技术有了进步, 但由于研究为回顾性、样本量小和最重要的随访时间短, 研究结果不尽人意。本综述旨在深度探讨经巩膜缝线固定人工晶状体植入术的进展, 并对今后的发展方向提出建议。
经巩膜缝线固定的眼内人工晶状体植入术——手术技术的演变与未来发展
摘要
在缺乏晶状体悬韧带或囊膜支撑的情况下,二期人工晶状体植入有多种选择。在复杂性白内障手术、外伤或遗传性疾病(如马凡氏综合征或假性脱落)的白内障手术中,可能会出现晶状体囊膜缺失的情况。而光学治疗措施(佩戴框架眼镜或隐形眼镜)以及手术方法(结合使用前房、虹膜固定或巩膜固定的人工晶状体)可以解决问题。自20世纪80年代首次发表关于巩膜内固定术的文章以来,植入经巩膜缝线固定人工晶体的外科技术经历了各种各样的改进。然而,尽管外科手术技术有了进步,但由于研究为回顾性、样本量小和最重要的随访时间短,研究结果不尽人意。本综述旨在深度探讨经巩膜缝线固定人工晶状体植入术的进展,并对今后的发展方向提出建议。
Similar content being viewed by others
Introduction
Intraocular lens (IOL) implantation in the absence of zonular or capsular support can be accomplished by the implantation of an anterior chamber intraocular lens (ACIOL), iris-fixated lens, or scleral-fixated intraocular lens (SFIOL). The loss of this support occurs most commonly in the context of complicated cataract surgery involving compromise of the anterior and/or posterior lens capsules [1, 2] but can also occur after zonular dehiscence arising from ocular trauma, pseudoexfoliation syndrome, and inherited conditions (e.g. Marfan syndrome and Homocysteinuria).
ACIOLs were initially of the closed-loop configuration and resulted in a high incidence of progressive corneal endothelial cell loss, persistent inflammation and secondary glaucoma, as well as cystoid macular oedema (CMO). This however has changed since the introduction of open-loop ACIOLs, which have a much lower rate of these complications with an adequate safety profile [3] and are more commonly used in the modern era of aphakic secondary lens implantation [4]. Iris-fixated lenses can be sutured or sutureless. Various techniques of suturing the IOL haptic and optic to the iris have been extensively described [5]. In recent years, sutureless ‘iris claw’ lenses have been more widely accepted in the setting of aphakia with loss of zonular or capsular support. In brief, the surgical technique involves enclaving a small knuckle of iris tissue in the “claw” in a groove on each side of the Artisan or Verisyse IOL. A recent study comparing the outcomes of anterior versus retropupillary iris-claw lenses in aphakic eyes found that both techniques were effective in improving vision, with a low rate of intraocular complications [6]. However, a recently published retrospective study looking at 24-month follow-up of retropupillary iris-claw lenses found a disenclavation rate of 9.4% [7] whereas a previous study reported an even higher rate of 14% over a period of 6.7 months [8]. Generally, it is accepted from clinical and histopathological studies that posterior chamber intraocular lenses (PCIOLs) offer several advantages over anterior chamber lenses. A well placed PCIOL may reduce the risk of corneal endothelial loss, damage to angle structures, pupillary block glaucoma and iritis, compared to ACIOLs. In addition, the optical properties of PCIOLs are possibly better, as there is a theoretical basis that the IOL can be positioned closer to the nodal point and centre of rotation of the eye [9].
The success of iris-fixated implants depends on a structurally normal iris, anterior chamber, and angle status. Additionally, the longer-term impact of such implants on anterior chamber endothelial reserve remains uncertain. Therefore, SFIOL implantation is particularly important in the surgical correction of aphakia in patients with pre-existing anterior chamber abnormalities, glaucoma and iris defects (e.g. aniridia), and may therefore be indicated in eyes with compromised corneal endothelium [10]. In addition, a history of uveitis may preclude the use of a secondary lens that increases the risk of iris chaffing such as an anterior chamber or iris-fixated lens and there are reports of good visual outcomes following the use of SFIOL implants in patients with pre-existing uveitis [11,12,13]. Over the years, several techniques have been adopted for the fixation of IOLs to the sclera, including both sutured and sutureless approaches.
This review article will look at the main techniques for sutured and sutureless SFIOLs to explore whether there is an optimal technique favouring better visual and refractive outcomes. In addition, it will look at the outcomes and complication rates of current published techniques.
Search methodology
A literature search was conducted in PubMed between the years 1985–2021 for the words “scleral-fixated intraocular lens”, “transscleral intraocular lens”, “intrascleral intraocular lens”, “sutureless posterior chamber intraocular lens”, and “glued intraocular lens”, yielding a total of 1254 articles. Any article in a language other than English, studies in animal models or those combining surgical procedures of different subspecialties (i.e. penetrating keratoplasty with scleral-fixated IOL) were not considered. The remaining abstracts were reviewed by three authors (SMS, BFS and RA) and were included based on their relevance to the review article. Case series with less than 20 patients were not included unless the authors felt they contributed to the topic substantively. This was decided on an individual basis after reviewing each of these articles.
Non-surgical management
The decision to perform surgery versus to manage conservatively should be based on individual needs. IOL implants can provide long-term vision stability, but surgical procedures are not risk-free. In contrast, aphakic correction with spectacles or contact lenses avoids possible intraoperative and postoperative vision-threatening complications; this treatment option could be suitable in cases where the patient is deemed to possess pre-existing risk factors for a poorer outcome or limited visual prognosis. However, non-surgical correction is not always well tolerated and may preclude functional vision improvement, especially in monocular aphakic patients.
After the crystalline lens has been removed, the focal plane will form behind the eye causing a state of marked hyperopia. To be able to correct this refractive error, a high-plus spectacle lens is needed to bring light rays focused on the retina. The high-plus spectacle lens induces a prismatic effect by bending light rays progressively towards its edge. This effect creates a blind circle-shaped area between 51 and 64 degrees from fixation on the temporal visual field—phenomenon known as “ring scotoma”. The rest of the temporal field remains unrefracted and it is considered to be practically non-functional to the aphakic eye due to extreme blurriness. As the eye dynamically rotates to one side, the blind area induced by the edge of the spectacle lens shifts towards the centre, making peripheral objects to appear and disappear abruptly as the eye turns to fixate them directly (“Jack-in-the-box” effect) [14].
Additional negative effects of aphakic spectacle correction include: (a) excessive image magnification—which results in objects falsely perceived as closer (false depth perception) and falsely projected wider in the visual field (false projection); (b) distortion of the object shape—pincushion effect (images appear larger and elongated) and concave contraction of the field (peripheral field is perceived inwardly curved, e.g. hollowed room walls); (c) optical aberrations—spherical, comatic, chromatic and radial astigmatism; (d) altered near vision—secondary to increased convergence requirement and restricted field of view; (e) inaccurate prescription—due to difficulty in measuring the vertex distance and ocular changes during refraction process; (f) cosmetic issues of thick lenses [15].
Contact lenses offer better quality of vision by clearing up the optical defects produced by aphakic spectacle lenses. Potential risks and benefits should be weighed up carefully before initiating wear, and the patient needs to be instructed clearly about appropriate contact lens hygiene and wearing schedules. Younger patients tend to comfortably handle contact lenses, while older ones might struggle with its manipulation because of associated tremor, poor coordination or dexterity, and lack of support. Most of the complications associated with contact lens wear are self-limited; these include epithelial erosions, conjunctival papillary reaction, superficial corneal neovascularization, impaired lubrication and corneal hypoxia or oedema. Nevertheless, some may potentially cause permanent vision loss, such as trauma and microbial keratitis, which are less common in the elderly and in female patients. Meticulous patient selection, appropriate choice of contact lens material and precise fitting are crucial to successfully achieve extended contact lens wear and minimise rate of complications [16].
Surgical management
Preoperative considerations
Sutured vs. sutureless
There are a number of preoperative considerations when deciding to implant a SFIOL. SFIOL implantation is technically challenging, and there are a number of strategies and modifications which have been published in the literature. Suturing a SFIOL can be technically more difficult than sutureless fixation, increasing surgical time and, therefore, surgical expertise becomes an important factor in deciding whether to employ a sutured or sutureless technique. However, in certain situations, for example, involving trauma to the sclera or the limbus, it may be necessary to adopt a sutured technique to give better stability to the IOL [17].
Anterior vs. posterior segment surgeon
SFIOL implantation has been carried out by both anterior and posterior segment surgeons. Posterior segment surgeons are more likely to perform a pars plana vitrectomy (PPV) whereas an anterior approach involves an anterior vitrectomy prior to IOL implantation. A retrospective study comparing the two approaches found that visual improvement was similar in both groups, however, a myopic shift and IOL capture were more common with a PPV approach, whereas intraocular pressure elevation and IOL dislocation were more common with an anterior approach [18]. An advantage of the posterior approach would be to better visualise any posterior pathology and deal with any intraoperative complications (e.g. choroidal haemorrhage) more promptly, particularly in cases where lens dislocation is secondary to traumatic ocular injuries or complex cataract surgery.
IOL rescue versus IOL exchange
Another important consideration in patients who are pseudophakic is whether it is possible to ‘rescue’ a pre-existing dislocated or displaced IOL or whether an IOL exchange procedure needs to be performed. In-the-bag IOL dislocation involves the IOL-capsular bag or IOL-capsule tension ring-capsular bag complex and occurs years after uneventful cataract surgery due to progressive loosening of zonules with age or as a result of trauma, pseudoexfoliation syndrome, connective tissue disease, axial myopia and a history of vitreoretinal surgery. In comparison, out-of-the-bag dislocation is generally a result of complicated cataract surgery and occurs in the early postoperative period following cataract extraction. It is particularly important to consider the type of scleral fixation the surgeon is going to employ (i.e. transscleral suture fixation or sutureless intrascleral fixation) to determine if the original implant is suited to the surgical technique. A number of studies have looked at the outcomes following IOL rescue and refixation as well as comparing refixation with lens exchange to determine the most appropriate option for scleral fixation [19,20,21,22,23,24].
Can et al. [14] reported good short-term results with the use of a single-piece acrylic lens sutured to the sclera after securing the lens with a hitch-cow knot around each haptic. However, concerns regarding iris-touch and lens stability in the long term with a lack of specific one-piece lens designs for scleral fixation means that these are not widely used for SFIOL implantation. A retrospective, comparative study that compared two different surgical techniques to rescue dislocated IOL implants and fixate them with either vicryl sutures or fibrin glue reported no postoperative IOL dislocations or tilt and an improvement in overall visual acuity [20]. Another retrospective study comparing IOL refixation to lens exchange using perfluorocarbon liquid and fibrin glue found no difference in visual outcomes and complications between the two groups [21]. In addition, a recent comparative study [24] looking at IOL refixation versus IOL exchange found more favourable visual outcomes with refixation as well as reduced vitreoretinal complications when compared to IOL exchange. However, both studies were limited by a short follow-up period of 6 months. Two randomised trials that compared IOL repositioning with scleral suturing to IOL exchange by retropupillary iris-claw for in-the-bag IOL dislocations found no statistical difference in visual outcomes and safety profile between groups, and concluded that both methods are suitable for treating in-the-bag IOL dislocations [23, 25].
Scleral-fixated intraocular lens in paediatric eyes
SFIOLs in the paediatric population are usually a result of lensectomy in early childhood, after trauma, or in the setting of lens subluxation from hereditary disorders [26]. In contrast to secondary IOL implant in adults, amblyopia and visual rehabilitation play a fundamental role in this subgroup of patients who require close and careful follow-up, in order to avoid long-term visual loss.
Aphakia during infancy can result in amblyopia, however, conservative management with spectacles or contact lenses allows us to achieve good visual outcomes, postponing the need for surgery [27]. Sutured scleral fixation has been the preferred technique accounting for the majority of publications. Sen et al. [28] described surgical outcomes and complications of sutured SFIOLs in 279 paediatric eyes with traumatic cataract or congenitally subluxated lens. Mean age of patients was 10 years. All patients underwent vitrectomy and sutured scleral-fixated IOL (single-piece PMMA posterior chamber IOL with 2 eyelets, Hanita lenses) using 10–0 polypropylene suture and a two-knot four-point ab externo fixation technique. They report an increase or maintenance of best corrected visual acuity (BCVA) in 93% with a mean of follow-up of 39 months. Retinal detachment and endophthalmitis were reported in 5.7% and 0.7% respectively. The same author reported similar results with the use of sutured SFIOls in 73 paediatric eyes with Marfan syndrome [29]. A single-piece PMMA PCIOL was introduced through the scleral tunnel after the passing of 10–0 polypropylene sutures through the haptic eyelets, and with four-point suture fixation. Retinal detachment was described in three cases and IOL dislocation/subluxation was noted in 5 eyes.
In terms of sutureless intrascleral fixation, the use of fibrin glue has been described by Singh and colleagues [30] in 41 eyes using partial thickness scleral flaps with one or three-piece IOL and fibrin glue. In this series neither retinal detachment nor endophthalmitis were observed during a mean follow-up of 17 months. Kannan et al. [31] evaluated refractive outcomes and complication profile of sutureless, glueless, flapless, intrascleral fixation of IOLs in 40 paediatric eyes. They used a standard three-piece nonfoldable IOL (Aurolab, Madurai, India) with the haptics tucked into the scleral pockets. One patient developed inferior subluxation of IOL. No retinal detachment or endophthalmitis were reported by this group. The median follow-up was 12 months (range, 12–62 months).
Surgical techniques of sutured scleral-fixated iol implants
Over the years, there have been several different approaches to sutured scleral fixation of IOLs [9, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. These have varied mainly in terms of: the use of an ab interno or ab externo approach for suture introduction, the number of points of IOL fixation, suture material, and the methods to avoid suture erosion [55]. Table 1 summarises the various studies that looked at visual outcomes and complications following transscleral sutured IOL fixation techniques.
Ab externo vs. ab interno approach
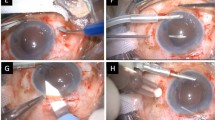
The advent of scleral fixation techniques began with suturing of IOLs placed within the sulcus. An ab interno approach involves passage of the sutures via a needle from inside the eye through the scleral wall. In contrast, the ab externo technique involves passing the needle from outside to inside. One of the first methods for sutured IOL fixation was described by Malbran and co-workers where a sutured SFIOL was implanted following an intracapsular cataract extraction. Suture loops were introduced into the eye in an ab externo fashion with a 28-Gauge needle and the haptics fixed to the sclera at the 3 and 9 ‘o’ clock positions, 2 mm from the limbus [56]. Lewis and co-workers further popularised the ab externo technique, where sutures were introduced through the creation of a scleral flap and the knot was buried in the bed of the flap to secure the implant (Fig. 1) [57].
A A superior conjunctival peritomy and partial-thickness scleral flaps are performed opposite to each side of the limbus, preferably avoiding the 3 and 9 o’clock positions to spare the ciliary vessels (e.g. 2 and 8 o’clock for right eyes and 4 and 10 o’clock for left eyes). Then a corneoscleral wound is created and complete anterior vitrectomy is carried out. After filling the anterior chamber with viscoelastic, a straight needle conveying 10–0 polypropylene is inserted through one of the scleral flaps parallel to the iris at 1.5 mm posterior to limbus, until it is visualised. A 28-Gauge needle mounted on an insulin syringe is inserted in a similar manner through the second scleral flap; the suture needle is docked into its lumen. B The 28-Gauge needle is withdrawn from the eye carrying the suture, which should be visualised crossing the anterior chamber from sulcus to sulcus. C Using a Sinskey hook, the suture is pulled out through the corneoscleal wound and then cut in half. D The free ends of the suture are tied to each one of the haptics of a PMMA IOL. Lens forceps are used to introduce the IOL into the anterior chamber through the corneoscleral incision and the tension of the sutures is adjusted symmetrically until the desired position is reached. E A second suture is used to take a short bite in the scleral bed anterior to the exit of the first suture; both are incorporated into a square knot. To finalise, the scleral flaps and conjunctival peritomy are closed.
Smiddy et al. described an ab interno technique where the suture loops were passed from inside the eye to the outside using a small gauge needle. Although they did not report any major complications with the technique through the use of a corneoscleral incision, the main issue with this technique was the blind passage of the needle 1 mm posterior to the limbus from within the eye. The authors advocated employing anterior vitrectomy as a standard in all patients prior to the placement of the sutures in order to avoid vitreous incarceration and migration into the anterior chamber, which was seen in the first few postoperative cases [9]. Hadayer et al. described an ab interno technique of suturing a foldable one-piece (hydrophilic acrylic) IOL using Gore-Tex sutures by injection through a 2.4 mm corneoscleral incision. The leading haptic is held in the anterior chamber with a 27-Gauge forceps while another pair of forceps is used to pass the suture through the haptic eyelets inside the eye, before it is externalised and tied [58].
Avoiding suture erosion and exposure
Scleral flaps and burial of knots
Traditionally, techniques to secure IOLs to the sclera relied on tying a polypropylene suture to the haptic of the lens and externalising it through the sclera. This ignored the hazards of an externalised suture knot, which was covered by conjunctiva alone leading to the obvious issue of exposed suture knots and the risk of endophthalmitis. A refinement of this technique came with the creation of partial thickness scleral flaps to protect the knots which were then covered by the flaps to reduce the risk of knot erosion and exposure. Solomon et al. [32] published a technique which involved the creation of triangular scleral flaps at the limbus to cover the 10–0 polypropylene suture knot that was used to secure the IOL. The retrospective study revealed a 73% knot erosion rate through the sclera and a 17% erosion rate through the conjunctiva within an average of 9.4 months and 12 months, respectively. The study concluded that although creation of partial thickness scleral flaps delayed the erosion of suture knots, they did not prevent this from occurring. This has led to further modifications to transscleral IOL fixation techniques in order to improve rates of suture exposure and erosion. The addition of islets to haptics allowed the development of techniques to bury the suture knot within the eye, dramatically reducing the risk of knot erosion and exposure. Lewis et al. modified their original scleral flap technique to incorporate flap free suture fixation an additional suture pass through the sclera posterior to the limbus enabled the suture knot to be turned and buried into the eye. The retrospective study involved 100 patients, 60 of which had scleral flaps and 40 had the latter technique without the flaps. The average follow-up in the scleral flap group was 21 months and the flap free group was 15 months. 20% (n = 12) of patients with the scleral flap had knot migration and exposure of the suture within 8 months of follow-up. 80% of these patients (n = 10) required further surgery. In the remaining 40 patients without the scleral flaps, there were no identifiable cases of suture exposure [59].
Hoffman pocket
Hoffman et al. [60] described a technique of creating scleral pockets without the need for conjunctival dissection. Two opposing clear corneal incisions are made at the limbus with posterior dissection to create scleral pockets. A paracentesis is created anterior to each corneal incision to enter the anterior chamber. A double-armed 10–0 polypropylene suture is introduced through the paracentesis passed through the IOL haptic at the opposite end and externalised via a 27-Gauge docking needle through the scleral pocket. Once this has been repeated at the opposite end, the loop is externalised through the corneal paracentesis and tied. The knot is buried within the scleral pocket. A retrospective study by Yeung et al. looked at the visual outcomes and complications following implementation of Hoffman pockets to bury the scleral knot after transscleral fixation of a single-piece foldable IOL. Of the 40 eyes retrospectively reviewed, they did not report any major intraoperative complications with self-limiting postoperative complications, namely mild vitreous haemorrhage, microhyphaema and transient elevation intraocular pressure. 8% of eyes developed CMO and 3% of eyes had decentration of the IOL at 2 months. There were no reported cases of knot exposure or endophthalmitis. Patients were followed up for a mean duration of 6 months [42].
Knotless Z-suture technique
Szurman et al. described a knotless technique using a zig-zag pattern (Z-suture) to secure the IOL to the sclera. This was achieved by reintroducing the suture close to its initial exit point from the eye and repeating the manoeuvre 5 times. The suture is then cut at the level of the sclera without a suture knot, precluding the need for a scleral flap or knot burial. The technique was performed in a total of 45 eyes. A transient ciliary haemorrhage was observed in three eyes. The implant was firmly attached within the sulcus in all cases, with a mean follow-up of 22 months. There were no reported cases of IOL tilt, suture erosion, loosening or atrophy [34]. However, another study to employ the Z-suture technique using 9–0 polypropylene with a longer mean follow-up duration of 64 months, reported an IOL dislocation rate of 13.8%, which was comparable to the reported incidence of this complication in previous studies (0–28%) [46]. Furthermore, another long-term retrospective study to evaluate the outcomes of the Z-suture technique revealed a suture breakage of 16.7% at 7.4 years and estimated the probability of suture breakage at 40% over a 10 year period. In addition, at a medial follow-up of 64 months, the risk of retinal detachment and CMO was 15.2% and 9.1%, respectively [44].
Points of IOL fixation to sclera
IOLs can be sutured to the sclera with 1, 2, 3, 4 or 6 fixation points [61, 62]. The original technique described by Malbran employed a two-point fixation [56]. Since then, various studies have been published with different points of fixation to improve stability and centration of the IOL as well as to improve refractive outcomes.
Young et al., developed a four-point suture fixation technique using 10–0 polypropylene sutures. A single-piece polymethyl methacrylate (PMMA) lens is inserted into the sulcus and the knots tied 2 mm from the limbus, rotated and buried into the sclera followed by conjunctival closure. The authors did not identify any case of suture-related problems such as erosion, kinking or exposure during a mean follow-up period of 18.3 months. Mild vitreous haemorrhage was noted in 20% of cases, with 10% incidence of limited choroidal detachment. Both these complications resolved without requiring any further intervention [50]. Another study looking at outcomes of the four-point fixation technique with the same lens identified 104 eyes which had undergone the procedure with a range of follow-up between 12 and 180 months (mean = 73.4 months) [35]. The complications noted were exposed suture knots (3.8%), corneal decompensation (4.8%), subluxed IOL (1.9%), retinal detachment (1%), choroidal detachment (1%) and tilted IOL in (1%). Overall, 12.5% of eyes required further surgery to deal with postoperative complications. The rate of suture related complications was much lower in this study (3.8%) compared to previous studies which have reported rates as high as 28–57% [32, 51].
Fass et al. [63] used a specially designed foldable one piece hydrophilic acrylic lens (Akreos®, by Bausch & Lomb) with a four-point fixation technique using a 2.75–3.0 mm corneal incision. The surgical technique was that described by Hoffman et al. [60] in 2006 which involved passage of a double-armed suture through the scleral pocket and conjunctiva without creating a conjunctival peritomy. Their outcomes were published in a subsequent study of nine patients who were implanted with a hydrophilic acrylic lens, with a mean follow-up of 3.4 months. Four eyes (44%) had a vitreous haemorrhage, all of which resolved spontaneously. Two eyes (22%) had mild choroidal effusions which settled within 3 weeks, lens decentration occurred in two eyes (22%), both of which required re-centration. One eye (11%) had high intraocular pressure and corneal oedema. There were no reported cases of CMO or pigment dispersion [33]. Almashad et al. [61] reported a four-point scleral fixation technique without the use of scleral flaps with 10–0 polypropylene sutures in 15 eyes with a follow-up of 6 months. They reported a high incidence of ciliary bleeding during needle passage (40%), although no cases of suture breakage/erosion, IOL decentration, retinal detachment or endophthalmitis were seen. There have been recent case series reporting opacification of the acrylic Akreos® lens after intraocular tamponade with both gas and silicone oil. This is particularly concerning in this cohort of patients who are at higher risk of retinal detachment that may require further surgery with intraocular tamponade [64, 65].
A recent study employed a six-point suture fixation technique without the need for conjunctival dissection. A double-armed 8–0 polypropylene suture was used with a 3-looped haptic, single-piece, hydrophilic acrylic foldable lens (Zeiss CT ASPHINA 603P). They performed the technique on 21 patients with a mean follow-up of 7.8 months. Four patients (19%) had transient corneal oedema and three patients (14.3%) had transiently elevated IOP. They did not identify any cases of suprachoroidal haemorrhage, vitreous haemorrhage, retinal detachment, suture breakage or knot exposure, IOL tilting or decentration. They found that the closed-loop haptic design of the lens meant that the risk of knot slippage was not an issue and the three loops gave better fixation and centration [62].
Suture material
Traditionally, 10–0 polypropylene sutures were used for the various scleral-fixated techniques (as described above). In order to improve durability and reduce risk of suture breakage, other suture types have been used, such as 9–0 polypropylene and 8–0 Gore-Tex [2]. The Gore-Tex suture has been used in cardiothoracic surgery for over 20 years with minimal evidence of suture breakage over time and has been reported to have excellent tensile strength improving the stability of the suture [66]. Khan and co-workers used a single-piece PMMA lens (Alcon CZ70BD) and a one-piece hydrophilic acrylic foldable lens (Akreos A060) with 8–0 Gore-Tex suture material. The initial technique used a 23-Gauge system with a high rate of postoperative hypotony (9.4%), which improved significantly by employing a 27-Gauge system (n = 0) (Fig. 2). Interestingly, there were no cases of suture breakage with either gauge with a mean follow-up of 11 months (85 eyes) in the first study and 4 months (eight eyes) in the second study. However, the duration of follow-up was short, limiting the ability to draw any definitive conclusions [38, 39, 67]. Another retrospective study of 20 patients who underwent a four-point fixation with the Akreos lens showed favourable improvement in visual outcome (p < 0.0001) with minimal postoperative complications. There was no evidence of IOL tilt or decentration, but this was only performed on 6 of the 20 eyes and the mean duration of follow-up was only 8.9 months [45]. A study from China, using 10–0 polypropylene sutures with an S-shaped intrascleral suture reported no suture-related complications in 69 eyes, followed up for a mean duration of 34 months [47]. A prospective Spanish study reported favourable outcomes with the use of non-absorbable 10–0 polyester fibre (Mersilene) sutures. Of the 25 eyes, one patient needed resuturing after decentration of the lens. However, the study was limited by its small sample size and a mean follow-up of 18 months [68].
A Conjunctival peritomy in superonasal and inferotemporal quadrants is performed. The corneal surface is marked 180 degrees apart with a toric lens marker in the same location. Two scleral marks are created on each side 3 mm posterior to the limbus and 5 mm apart from each other, straddling the corneal marks. 27-Gauge vitrectomy trocars are placed at the superior marks and standard vitrectomy is carried out. Additional sclerotomies are created at the inferior marks using trocar blades parallel to the limbus on each side. B One half of the CV-8 Gore-Tex suture is threaded through each pair of haptics of the Akreos IOL, passing from anterior to posterior through the firs eyelet and then from posterior to anterior through the second eyelet on the same side. This manoeuvre is repeated for the contralateral pair of haptics with the other half of the suture. C, D A handshake technique with forceps is used to pass the ends of the suture from the leading haptics through a clear corneal incision, which are then retrieved through the corresponding sclerotomy one at a time. E After both ends of the suture from the leading haptics have been passed, the corneal incision is enlarged. Prior to insertion, IOL is manually folded using McPherson forceps underneath and 0.12 mm forceps above. The sutures from the leading haptics are gently pulled. F Once the IOL is in the anterior chamber, the Gore-Tex from the trailing haptics can be then retrieved from the corresponding contralateral sclerotomies using the same handshake technique. The 27-Gauge trocars are removed, and the Gore-Tex is tied using a slip knot or 3–1–1 technique on each side, ensuring a well-centred IOL. Knots are buried and peritomy is closed.
Late breakage of 10–0 polypropylene sutures has been highlighted in the study of Vote and co-workers on cases which underwent a two-point fixation technique and pars-plana vitrectomy [51]. This reported a suture breakage rate of 28% at a mean interval of 4 years after initial surgery. This followed up an earlier study from Moorfields using the same technique on a similar patient cohort with a mean of 20 months follow-up which reported good visual results (final acuity limited only by pre-existing pathology) and a low rate of complications—notably only one of 63 cases had suture breakage [69]. These reports emphasise the need for long-term follow-up of cases undergoing the varied techniques of lens implantation in the absence of zonular or capsular support. A parallel study from the Moorfields group, using the same implantation technique in non-vitrectomised eyes reported higher levels of complications suggesting that the interaction of sutured posterior chamber lenses with the vitreous may increase complication rates [70]. The indications for surgery and pre-operative co-existing pathology do, however, differ between these series and conclusions on comparisons must be treated with caution. A similar study by Bading et al. [52] looked at the long-term outcomes of scleral fixation following the use of 10–0 polypropylene sutures with scleral flaps. In contrast to the study by Vote et al., this retrospective study with a mean follow-up of 43.5 months had a much lower rate of IOL subluxation (6.3%); however they did find a similar rate of retinal detachment (6.3% vs. 8.2%). The authors concluded that despite its retrospective nature, sutured IOL fixation with 10–0 polypropylene sutures was a safe and effective option for secondary IOL implantation in young patients.
Sutureless scleral-fixated intraocular lens
Initial techniques
With increased numbers of reports of various techniques describing sutured lenses, it emerged that there were a range of common complications, including suture erosion, endophthalmitis and IOL decentration. This led Scharioth et al. from Germany, to describe the first sutureless technique to anchor SFIOLs to the sclera. The authors used an anterior approach that involved burying of externalised haptics into scleral tunnels. Their initial publication included five patients, none of which were reported to have major complications in the first 3 months of follow-up [71]. A further analysis of the study technique was combined as part of a multicentre trial in 2010, involving 63 patients across four European institutions. They reported complications of transient corneal oedema (7.9%), persistently raised intraocular pressure (3.2%), IOL dislocation (3.2%), vitreous haemorrhage (3.2%) and iris capture (2%). Three patients required further surgery to stabilise the lens. None had visual outcomes which were worse than that recorded preoperatively. It was concluded that sutureless lens fixation had favourable visual outcomes and lens tilt can be minimised by ensuring the implanted 3-piece IOL has a haptic design the full diameter of the ciliary sulcus. However, the median follow-up period was 7 months and the study was retrospectively conducted, limiting the conclusions from the study [72]. A modification of this technique using 23-Gauge trochars with a posterior approach was described by Prenner et al. [73] in 2012. One-year outcomes of the technique reported IOL dislocation in 12% of patients, although there was a statistically significant improvement in visual acuity. However, they had a small sample size of 24 patients [74].
Glued IOL technique
Agarwal and team described a novel technique of sutureless SFIOL implantation with the use of quick-acting fibrin glue derived from human plasma. It involved the creation of scleral flaps and externalisation of haptics into the flap before it was glued closed [75]. They included 53 eyes in their analysis and reported IOL decentration in 5% of cases over 1-year, with CMO in 7.5% of eyes [76]. A modification of this procedure described as the “handshake” technique was described in 2013 by the same group, to reduce problems related to haptic externalisation [77]. A further modification of this technique by Beiko and Steinert suggested the use of a silicone tire to maintain the externalised haptic, thereby nullifying the need for an assistant [78].
The first North American series implementing the glued IOL technique was published by McKee et al. [79] in 2014. They primarily followed the Agarwal technique described above, with slight modifications, to include more robust haptic material in the IOL and the lifting of only two edges of the scleral flap. This allowed a more reliable creation of the scleral tunnel, strengthening its anterior aspect. They included 50 patients in their series, with a high initial overall rate of transient hypotony (22%), with one patient needing a second procedure to seal the leak. This was attributed to the learning curve associated with SFIOL implantation using glue. They also had two cases of transient vitreous haemorrhage which resolved spontaneously. One eye needed repeat surgery for a broken haptic-optic junction, 3 months following initial surgery. The largest outcome data for the glued technique included 735 eyes with a mix of rigid and foldable IOLs and a mean follow-up of 16 months. The retrospective study showed an improvement in visual acuity for both types of lenses. Amongst the 191 eyes with foldable IOL, the noted postoperative complications were optic capture (5.7%), IOL decentration (2.6%), uveitis (0.5%), haptic displacement (2%) and haptic extrusion (0.5%). Of the 486 eyes that underwent a rigid IOL implant, the complications included optic capture (3.4%), uveitis (3%), IOL decentration (4.3%), haptic displacement (4.1%) and retinal detachment (1%) [80, 81].
‘Double-needle’ or Yamane technique
One of the most popular sutureless techniques in recent times was proposed by Yamane and co-workers [82]. The technique involved the use of 27-Gauge needles to externalise both haptics simultaneously from the eye in a ‘double-needle’ method, which were then embedded into limbal tunnels into the end of partial thickness scleral dissections (Fig. 3). The initial study of 35 eyes showed no evidence of IOL dislocation at a mean follow-up of 10 months. IOL capture was seen in 9% of cases within this short period. Interestingly, the study found a mean IOL tilt of 2.3o, which was not significantly different to IOL tilt noted after ‘in the bag’ implantation following routine cataract surgery. A modification of the technique by Yamane’s team involved the use of cautery to create ‘flanged’ fixation of the IOL haptics without scleral dissection [1]. Of the 100 eyes that underwent this technique, there was a statistically significant improvement in visual acuity up to 24 months following the procedure. The mean IOL tilt was 3.4o, with a mean refractive difference of −0.2 + 0.99 D. Postoperative complications included iris capture (8%), vitreous haemorrhage (5%), hypotony (2%) and CMO (1%). There were no reported cases of retinal detachment, IOL dislocation or endophthalmitis. Smaller studies with minor modifications of the technique have reported no significant complications, albeit very short follow-up periods and small sample size [83, 84].
A Having performed prior vitrectomy, a 3-piece IOL is inserted into the anterior chamber using an injector through a clear corneal incision, leaving the trailing haptic outside the wound. A 30-Gauge thin-wall needle is used to create an angled sclerotomy through the conjunctiva, 2 mm posterior to the limbus. B The leading haptic is passed into the lumen of the needle using forceps. C A second sclerotomy is made with a 30-Gauge thin-wall needle at 180 degrees from the first one. The trailing haptic is inserted into the lumen of the second needle using forceps, while the first needle is still in place. D The needles are used to externalise both haptics onto the conjunctiva simultaneously. E The ends of the haptics are cauterised, and each flange is pushed back to be anchored into the scleral tunnels.
Trochar technique
Todorich et al. described a transconjunctival approach for transscleral fixation using 25-Gauge or 27-Gauge trochar cannulas 2 mm posterior to the limbus 180o apart, through which a 3-piece foldable (Alcon MA60AC) lens injected into the anterior chamber is externalised. 27-Gauge maxgrip forceps are used to externalise the haptics through the sclerotomies and positioned under the conjunctiva [85]. A recent large retrospective analysis looked at 122 consecutive patients who underwent this technique and found that the commonest complication was vitreous haemorrhage (22%). The mean follow-up was 1.5 years. Spontaneous resolution of the haemorrhage occurred in 67% of cases. The incidence of IOL decentration, dislocation, haptic erosion and breakage were reported as being low [86]. The authors also found that haptic flanging increased IOL stability compared with the traditional unflanged technique, with a mean follow-up of 6.5 months [87]. Although there is concern regarding haptic erosion of the conjunctiva with sutureless fixation, it is thought that tucking the flanged haptic within the scleral tunnel reduces this complication [55].
A novel IOL developed in Italy (the FIL SSF Carlevale IOL) is a foldable one-piece injectable lens that has specially designed transscleral plugs which, when externalised through the 25-Gauge trochar sites (180 degrees apart), are positioned over the scleral surface and covered by intact conjunctiva. This has the obvious advantage of a smaller corneal wound and potentially reduced haptic complications due to the novel design. However, current studies report on a very small number of patients with short term follow-up and longer-term data is required before a definitive conclusion on safety and risk profile can be made [88, 89].
Table 2 lists various studies that looked at visual outcomes and complication rates following sutureless SFIOL implantation over the years [1, 72, 74,75,76, 79,80,81,82,83, 86, 90,91,92,93,94,95,96,97].
Comparative studies
As described above, there are three main options for IOL implantation following loss of zonular or capsular support—ACIOL, iris-fixated and SFIOL implantation. A number of studies (Table 3) in the literature have looked at comparing these surgical options [24, 25, 98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117].
ACIOL versus SFIOL
Evereklioglu et al. compared the visual outcomes and complication rates between open-loop ACIOLs and single-piece SFIOLs [108]. They conducted a retrospective analysis of the two surgical approaches used to correct aphakia secondary to complicated cataract surgery with a mean follow-up of 34 months. Of the 124 eyes included in the study, 73 underwent ACIOL insertion and 51 eyes scleral-fixated with a single-piece PMMA lens, sutured with 10–0 polypropylene using scleral flaps. The BCVA was better in the SFIOL group, although the results did not reach statistical significance (p = 0.718). There was a higher incidence of intraoperative anterior chamber haemorrhage in the ACIOL group (6.8% vs. 3.9%), although no serious intraoperative complications were seen in either group. The most common early postoperative complication seen was a higher incidence of transient corneal oedema in the ACIOL group (9.6% vs. 5.9%), however, all cases resolved within 3 weeks. The main postoperative complication seen was CMO and occurred in 6.8% of ACIOL eyes, and 3.9% in the SFIOL group. Iris capture or pupil decentration was more common in the ACIOL group, however, IOL decentration/tilt occurred more frequently in the SFIOL group. Vitreous haemorrhage (2.0%) and suture erosion (7.8%) occurred exclusively in the SFIOL group. There were no reported cases of retinal detachment or endophthalmitis in either group. Overall, there was no statistically reported difference in complication rates between the groups during the follow-up period, however, given the short follow-up period it is not possible to comment on rates of longer-term complications such as corneal decompensation which may be a key consideration when comparing SFIOL implants to anterior chamber lenses. Similarly, another retrospective study with longer mean follow-up period of 63.5 months, did not find any statistically significant difference in visual outcomes or postoperative complications between ACIOL implantation and sutured SFIOL [113]. More recently, Khan et al. [114] compared outcomes of combined PPV with ACIOL versus SFIOL implantation using Gore-Tex sutures. Of the 63 eyes in the study, 33 eyes underwent ACIOL implantation and 30 eyes SFIOL implantation. Both groups showed a statistically significant improvement in visual acuity, although there was no difference between the groups at 1-year follow-up. In terms of postoperative complications, those who had an ACIOL implanted had a 30.3% incidence of transient corneal oedema vs. 6.17% in the SFIOL group.
Iris-fixated versus SFIOL
Kim et al. [116] compared outcomes of iris fixated IOL implantation against scleral-fixated IOLs in a retrospective case series. A total of 44 eyes underwent scleral fixation and 35 eyes underwent iris-fixated implants. The technique for scleral fixation involved an ab externo procedure with the use of 27-Gauge needle to externalise the sutures before burying the knot within the sclera. Visual outcomes were favourable in both groups after the procedure and were maintained for 12 months following surgery. Although there were no differences in overall refractive error between the groups, astigmatic errors were fewer in the scleral fixation group. The frequency of perioperative complications, such as intraocular bleeding, corneal endothelial loss, CMO and retinal detachment were similar in both groups. The frequency of immediate postoperative inflammation was higher in the iris-fixation group, but this was not significant with extended follow-up. The recurrences of inflammation were found to be earlier in the iris fixation group. Zheng et al. [115] compared iris-claw lens fixation to sutured SFIOL (with 10–0 polypropylene within scleral flaps) in a randomised case series involving 71 patients with Marfan syndrome. Interestingly, although the visual outcomes were similar between the groups, the rate of IOL decentration was much higher in the SFIOL group (48.7%) compared to none in the iris-claw group.
Vounotrypidis et al. [100] conducted a large retrospective study looking at 174 eyes which underwent secondary IOL implant for aphakia with either an ACIOL, iris fixation, sulcus fixation or scleral fixation. The majority of patients had an ACIOL implanted and only 12 eyes underwent a scleral fixation implant. Of these, two eyes in the SFIOL group had an IOL dislocation (17%), whereas only one eye in the ACIOL group dislocated (2%). All techniques showed similar visual and refractive outcomes [63].
Sutured versus sutureless SFIOL
In recent years, studies have looked at comparing sutured against sutureless SFIOL implantation techniques. Ganekal et al. [101] compared visual outcomes and complications after suture-assisted and fibrin glue-assisted SFIOL implantation. They included 50 patients in the study, 25 in each group. The two groups had similar visual outcomes at 6 months, with 20/40 or better vision in over 80% of patients. The rate of postoperative inflammation was 48% in the sutured group vs. 16% in the glue-assisted group. In addition, glaucoma was also more common in the sutured group (40% vs. 16%). Haszcz et al. [102] looked at 42 eyes in a retrospective case series, 31 of which underwent scleral fixation using the Hoffman technique and 11 eyes using the sutureless Scharioth technique. Visual outcomes were similar in both groups. Two patients who underwent the Sharioth technique had IOL dislocation at 2 months. Sight threatening complications of expulsive haemorrhage, retinal detachment, prolonged inflammation or glaucoma were not observed in either group with a mean follow-up of 14.5 months. Similarly, a study in India compared a four-point suture fixation technique to a sutureless Scharioth technique in 109 eyes and found similar results in terms of visual outcomes and postoperative complications in both groups, with IOL dislocation in one patient in the sutured group. The study was limited by its retrospective nature and a mean follow-up of 19 months [103].
Refractive outcomes
Modern day cataract surgery pays close attention to refractive outcomes, to enable patients to obtain spectacle independence. Nevertheless, secondary IOL implantation in the absence of zonular or capsular support poses a bigger challenge when predicting accurate refractive targets. Patients requiring a secondary IOL implant are usually those with previous history of ocular conditions which may alter visual function permanently. Refractive status will vary depending on several perioperative factors such as the technique of choice, surgeon’s expertise, and presence of intra or postoperative complications. Additionally, achieving a steady postoperative position of the IOL will ensure a stable refractive status in the long run. For example, any changes in the structures providing an anchor point for the IOL implant, such a loss of tensile strength of surrounding tissues or sutures, will determine the effective position and mechanical stability of the lens [118].
Brunin et al. [119] published results of 167 patients who underwent secondary IOL implantation in different locations: in ciliary sulcus with and without optic capture through capsulorrhexis, in the anterior chamber, fixated to the iris and sutured to the sclera. Predicted target refraction was calculated using Holladay 1 formula. Mean postoperative follow-up was 12 months. The following were the significant predictors that favoured postoperative visual outcomes in linear regression analysis: better preoperative visual acuity, absence of prior ocular pathology, IOL insertion in sulcus with optic capture, and absence of postoperative complications within 1 month after surgery (r = 0.604). Advantages of in sulcus fixation with optic capture included better IOL stability and reduced incidence of CMO in this subgroup. In this same series, refractive analysis of 114 patients showed no significant difference between all groups, with a median absolute refractive error of 0.50 D in each. This demonstrates variability in refractive outcomes between individual patients but a total low mean refractive prediction error. However, patients who required anterior vitrectomy (70%) showed a non-significant myopic shift (−0.28 D difference in refractive prediction error) when combining all groups (p = 0.06). Reasons behind this refractive change are not well understood, but similar outcomes have been observed after combined phacovitrectomy and in eyes undergoing PPV that are already pseudophakic [120, 121]. Proposed hypotheses include: (a) decreased effective lens position, especially when intraocular tamponade is used; (b) postoperative changes in anterior chamber depth; (c) replacement of vitreous gel with aqueous humour, and subsequent change in refraction index; and d) increased axial length following PPV [120, 121].
In a retrospective analysis of sutureless intrascleral IOL fixation and PPV, Lee et al. [122] compared refractive outcomes after placing a 3-piece aspheric IOL (MA60AC- Alcon in 81 eyes, or ZA9003—Johnson & Johnson in 3 eyes) inserted at 1.5, 2 and 2.5 mm from the limbus, using 25-Gauge or 27-Gauge trocar cannulas. Power calculation was performed with Barrett II Universal Formula; aim was to achieve plano to −0.50 postoperative spherical equivalent. Follow-up results at 3 months and 1 year showed stable mean postoperative spherical equivalent change from preoperative calculation of 0.58 ± 1.49 and 0.53 ± 1.21, respectively for all patients. Subgroup analysis revealed a hyperopic trend in patients with IOLs fixated further away from the limbus at 1 year, with a mean postoperative equivalent change of −0.33 ± 0.65 D at 1.5 mm, 0.48 ± 1.15 D at 2 mm, and 1.30 ± 1.39 D at 2.5 mm posterior to the limbus (p = 0.01). Given the stable refractive outcomes at 12 months, it was hypothesised that intrascleral fixation offers stability at 3 months and minimises long-term slippage through the creation of a steady tunnel in which haptics seat. One factor that might have influenced the refractive outcomes at increased posterior fixation from limbus was increased haptic tension, resulting in changes of the vault of 3-piece IOLs. Additionally, attention must also be focused on technique variations such as tunnel length, angle of entry and symmetry of haptic arrangement.
Su et al. [123] evaluated the refractive outcomes of 53 patients that underwent SFIOL implantation with Gore-Tex sutures. The type of lens used was a single-piece aspheric acrylic lens (Akreos A060 or enVista MX60). The lenses were sutured at either 2 or 3 mm from the limbus. The mean postoperative spherical equivalent was −0.99 + 1.00 dioptres (D), with mean deviation from target of −0.64 + 1.00 D. Subgroup analysis revealed that IOLs fixated at 2 mm from the limbus had a mean spherical equivalent −1.53 + 1.35 D, whereas those that were sutured 3 mm from the limbus had a mean spherical equivalent of −0.82 + 0.83 D. There was a statistically significant difference between the two groups (p = 0.09). The mean difference from target was also greater for the group with 2 mm fixation from the limbus (p = 0.03). The study concluded that at 2 mm fixation from the limbus, the refractive outcome was more myopic than at 3 mm, an important consideration when using sutured IOL implants. Similar myopic results of sutured SFIOL were found in another series by Kam et al. [124]. A total of 85 eyes underwent scleral fixation of a single-piece PMMA spheric IOL (CZ70BD—Alcon) with ab externo technique, using four points of fixation at 2 mm from limbus and 2 mm apart. Suture of choice was 10–0 polypropylene in 62 eyes, 9–0 polypropylene in 22 eyes and unknown type of suture in 1 eye. Formula of choice to calculate predicted refractive error was not mentioned; mean target refraction was −0.76 + 0.41 D. The main wound for IOL insertion consisted of a scleral tunnel, and 20% of patients underwent concurrent PPV. Only 66 eyes had absent concomitant ocular pathology and good visual potential. Mean spherical equivalent at 3 months in these eyes was −1.62 + 1.28 D, with 63% of them achieving final BCVA of >20/40. The effect of suture size was not explored.
Regarding transscleral IOL fixation with fibrin glue, Kumar et al. [118] recently reported long-term refractive outcomes in 91 eyes of 63 patients with capsular deficiency after at least 5 years of follow-up. Hydrophobic acrylic lenses were used in 94.5%, and the remaining corresponded to PMMA lenses. There was no mention of the formula used to calculate the IOL predicted refractive error. Surgical technique involved creation of limbal scleral flaps 180 degrees apart, and the IOLs were inserted through a 3 mm corneal incision or a scleral tunnel. Haptics were externalised through a sclerotomy incision made at 1–1.5 mm from limbus under the flaps and tucked into the intralamellar tunnel. All cases underwent anterior vitrectomy. Scleral flaps were closed with fibrin glue. There was no significant postoperative change from baseline (logMAR BCVA 0.61 + 0.7 preoperatively vs 0.5 + 0.50 postoperatively, p = 0.238). Mean postoperative spherical equivalent was −1.1 + 2.9 D and mean ocular residual astigmatism was 1.1 + 1 D. Clinically, minor IOL decentration of <0.5 mm was noted in only five eyes and it was not functionally significant.
Refractive results of the sutureless flanged intrascleral haptic fixation were reported by Rocke et al. [125] in 110 eyes undergoing insertion of a 3-piece IOL (Tecnis ZA9003—Abbott Medical Optics) and 25-Gauge PPV. Barrett Universal II Formula was used to calculate predicted refractive error. Primary outcome was the observed spherical equivalent at 1 month after surgery. Mean prediction error was +0.04 + 0.88 D. This outcome was compared with two additional groups, first one comprised of 100 concomitant patients undergoing routine phacoemulsification with in-the-bag IOL, and second one of 54 patients who underwent ACIOL insertion. When the mean prediction error was compared to the phacoemulsification group, results were not significantly different (−0.10 + 0.53 D, p = 0.185); however, when compared to the ACIOL group, the result was significantly more myopic (−0.76 + 0.89 D, p ≤ 0.001). Of these three groups, a significantly higher proportion of patients with a prediction error within +0.50 D were found in the phacoemulsfication (72%) than in the flanged haptic fixation (50%) or the ACIOL (34%) groups, p ≤ 0.001. The original technique reported by Yamane in 2017 [1] included also 100 eyes of 97 patients receiving four different models of 3-piece IOLs, with up to 36 months follow-up. Sclerotomies were made with 30-Gauge needles. The mean predictive error was 0.21 + 0.99 D for all groups, with no significant difference among them (p = 0.02). These results offer an insight regarding good visual outcomes offered by this technique with adequate haptic fixation thorough scleral tunnels and no use of adjuvants.
There are some important factors that must be taken into consideration while calculating biometry for intrascleral IOL insertion. Biometry correction factors should be applied whenever IOL calculation takes place in a pseudophakic eye, accounting for the refractive index of different materials and the central optical thickness [126]. Additionally, if there is an opaque structure blocking the optical centre of the eye (including a dense crystalline lens, a partially subluxed PCIOL or a fibrotic capsular remnant), an ultrasound biometry may be more suitable than an optical calculation. However, in cases with a clear optical centre, the preferred IOL calculation method is an optical biometry based on partial coherence laser interferometry to improve predictive value for postoperative refraction and precision of axial length measurements [127]. The use of optimised IOL A-constants, such as the ones provided by the ULIB site (User Group by Laser Interference Biometry) or surgeon-specific constants, are recommended to avoid systematic errors and to aim for greater accuracy [128]. Another important source of error in scleral-fixated lens cases is the final effective lens position. This will be determined by haptic location from the limbus, presence of residual peripheral capsular and lens matter that may displace the IOL, tightness of anchoring sutures, haptic tension, haptic angulation inducing changes in optic vault, and anatomical variations [128]. Unfortunately, none of these factors have been yet fully standardised. According to the recommendations of The Royal College of Ophthalmologists, postoperative spherical equivalent refraction benchmarks after routine cataract surgery include target predictive values within 1D in 85% and within 0.50 D in 55% of cases [129]. After insertion of an instrascleral IOL, these targets should be reproduced to the greatest extent.
Prevention of complications associated with scleral-fixated Iols
Early methods to fixate a PCIOL to the ciliary body and sclera were first described more than 30 years ago. Since then, there has been an improved safety profile of different IOL implantation techniques after surgeons have progressively acquired increased understanding of the possible associated complications and strategies to reduce their occurrence.
In general, a PCIOL fixated to the sclera offers some anatomical advantages compared to ACIOL insertion. Since a SFIOL is located more posteriorly, there is a higher hypothetical chance of preserving the integrity of the anterior segment by minimising damage to the corneal endothelium, disruption of the trabecular meshwork, narrowing of the anterior chamber or formation of peripheral anterior synechiae [130].
Corneal decompensation
Older ACIOL models with suboptimal size, vault and flexibility range caused many cases of bullous keratopathy [131]. However, implantation of any type of IOL can potentially cause endothelial cell loss. In a recent study, endothelial cell loss was quantified 2 years after retropupillary iris-claw fixation and IOL sutured to sclera. Results were not statistically different between groups (17.5%, n = 44 and 15.3%, n = 50; p = 0.15) and either procedure was concluded to be safe for any patient with at least >1500 cells/mm2 [132]. Careful manipulation of the anterior segment is warranted in any IOL implantation procedure, with ample use of viscoelastic to protect the corneal endothelium. This is especially important in patients with preoperative low endothelial cell density. In cases where corneal vulnerability is suspected (for example in advanced age, prior trauma or complicated surgery), a specular microscopy is mandatory to plan management alongside with a cornea specialist. Krause et al. [53] reported a 29% rate of postoperative corneal decompensation in patients undergoing scleral-fixated PCIOL insertion; most of these patients had history of ACIOL insertion and some level of preoperative corneal damage, which was the main indication for IOL exchange.
The rest of the series in this analysis reported mostly transient postoperative corneal oedema (0.5–10%), after sutured as well as sutureless techniques [47, 81]. This is one of the most frequently encountered early postoperative complications and is usually self-limited. Lee et al. [133] reported significantly higher rates of postoperative corneal oedema within 1 month of surgery in patients undergoing primary SFIOL simultaneously after complicated cataract extraction compared to those with secondary implantation (73% vs 44%, p = 0.027). These results highlight some of the risk factors for the development of this complication including prolonged surgical time, intense manipulation of anterior chamber structures, and iatrogenic corneal damage in some cases [134].
Wound leakage
Creation of optimal anterior segment surgical incisions requires appropriate selection of the entry site, optimal measurements and meticulous construction to avoid closure insufficiency and significant postoperative astigmatism. Foldable IOL implantation reduces the need of larger wounds and facilitates apposition of flap edges.
Wound leakage range is estimated between 1.6 and 8% [51, 52]. This early complication should be promptly recognised and addressed since it can lead to development of hypotony. The anatomical and visual prognoses are poorer if there is associated choroidal detachments [135]. Moreover, hypotony has also been linked as a risk factor to develop early reverse pupillary block, as reported by Higashide et al. [136] in three patients presenting after sutured PCIOL to the sclera with ab externo technique combined with PPV. The common findings included marked posterior bowing of the iris, abnormally deep anterior chamber and intermittent contact of IOL with pupillary rim, which were corroborated with anterior segment optical coherence tomography imaging. Two of these patients developed intermittent pupillary capture and required YAG laser iridotomy. An altered wound integrity during the episodes of reverse pupillary block was demonstrated. Another identified risk factor for this complication was pseudoexfoliation syndrome.
Ocular hypertension and glaucoma
Ocular hypertension is another commonly encountered early postoperative complication. It has been reported in up to 44% of cases following sutured PCIOL to the sclera [53]. It is more common in predisposed eyes with primary or secondary sclerocorneal angle abnormalities, traumatic zonular loss, established preoperative disease, and sensitivity to steroids [134]. It usually responds to topical hypotensives, but some patients continue to present raised IOP requiring medical or surgical management after 3 months of surgery. In this instance, it would be considered as postoperative glaucoma [137]. Additionally, there is a higher risk of developing glaucoma when hyphema develops or if there is prolapse of the anterior hyaloid. Vitreous remnants left in the anterior chamber could obstruct the trabeculum or induce persistent traction over other structures, resulting in secondary glaucoma [55]. This emphasises the need of careful concomitant anterior vitrectomy, if indicated, as well as adequate control of any sources of active bleeding intraoperatively.
Cystoid macula oedema
A scleral-fixated IOL theoretically acts as an anatomical barrier that avoids passage of vitreous strands or inflammatory mediators that could lead to chronic inflammatory processes [130]. Nevertheless, acute CMO has been reported in most sutured and sutureless techniques for IOL scleral fixation, occurring in up to 20–23% [49, 63, 86]. Risk factors include posterior capsular rupture and vitreous loss during initial complicated cataract surgery, history of uveitis, prolonged surgery or vitreous traction localised in the surgical wound or iris. It commonly presents during the first 3 postoperative months and responds well to topical steroidal and non-steroidal treatment. Prevention includes treating any pre-existing inflammatory reaction and planning surgery once it has settled, as well as minimising surgical manoeuvres and making sure to remove any points of vitreous incarceration.
Intraocular haemorrhage
Initial scleral fixation techniques, such as the ab interno method, involved more uncontrolled passage of needles behind the iris in the setting of hypotony and, with this, the possibility of inadvertent perforation of other intraocular structures such as the ciliary processes. With later implementation of the ab externo approach, the principle of keeping the anterior chamber closed minimised hypotony and reduced the risk of intraocular haemorrhage, vitreous incarceration in sclerotomy sites, and retinal detachment [134].
In the reviewed studies, rate of hyphema was reported to occur in up to 5% of cases and it was considered as a suture-related complication in two series in which PCIOL was fixated to the sclera using 10–0 polypropylene [42, 70]. This was classified as minor and was resolved spontaneously during the follow-up period. Vitreous haemorrhage ranged from 3 to 22% in this analysis [52, 86, 116]. It is usually mild and can be observed with adequate clinical control.
Suprachoroidal haemorrhage is an uncommon complication, but it has been reported to occur intraoperatively and up to 6 days after SFIOL implantation [138]. Risk factors include advanced age, uncontrolled systemic hypertension, and extreme axial length measurements [55]. Vote et al. [51] reported two cases of postoperative suprachoroidal haemorrhage among 61 eyes undergoing scleral-fixated IOL with 10–0 polypropylene; they reported a long-term risk of choroidal haemorrhage of 3.2% with this technique. Patients required drainage and tamponade with silicone oil.
Some principles to reduce the risk of acute intraoperative major bleeding include performing sclerotomies and suture passes at a safe distance from limbus to avoid the highly vascularised structures of the pars plicata, and keeping the globe well pressurised during surgery [55]. It is also important to minimise the number of puncture sites to the globe and to avoid direct trauma to major vessels of the iris. Efficient haemostasis of the scleral bed at the beginning of the surgery can also avoid blood diffusion into the anterior chamber. Late anterior or posterior segment haemorrhage can develop in cases where a poorly fixated IOL provokes continued chaffing against uveal tissues, leading to uveitis-glaucoma-hyphema syndrome [55, 134]. It can also present in patients with pre-existing complex ocular co-morbidities. Kansal et al. [94] reported a rate of 11.6% in patients with preoperative floppy-iris syndrome, trauma-related aphakia and prior retinal detachment surgery. This complication may also lead to secondary CMO in chronic presentations.
Suture and haptic-related complications
Placement of sutures through or directly below the conjunctiva yielded higher risk of erosions in the past [32]. More recent studies show a rate of 1.6–10% [48, 51]. To reduce risk of suture exposure, many methods now include rotation manoeuvres and suture burial under the sclera, as well as the possibility to create scleral flaps and pockets of different shapes and sizes to offer additional suture coverage [44]. It Is also important to avoid tying multiple knots when using thicker and more rigid sutures such as polypropylene, since these could erode even through scleral flaps [139]. Knotless techniques, such as the Z-suture technique, avoids risk of late erosion. Dimopoulos et al. [44] reported 0% rate of suture erosion with this technique in 62 patients after a follow-up period of 10 years.
Suture breakage is another concerning complication that could lead to IOL instability, especially when polypropylene sutures are used. Different mechanisms can lead to failure of the suture such as progressive thinning and cracking, enzymatic degradation induced by tissues with a high metabolic activity, or when the edges of haptics eventually cut the suture [40]. Suture breakage rate is variable; it has been reported in as high as 27.9% in a mean period of 4 years after surgery, while other authors have not reported it [38, 51].
Besides the structural support offered by the suture, it has been demonstrated that a fibrotic capsular matrix around the haptics forms after PCIOL sulcus implantation in 80% of cases, which accounts for additional IOL stability even in sutureless fixation techniques [140]. Wasiluk et al. [46] found an IOL dislocation rate of 14% after a mean follow-up of 64 months. All these cases occurred after breakage of 9–0 polypropylene; three cases occurred spontaneously, and 1 was secondary to trauma.
Improved haptic design has also allowed for better outcomes. For example, implementation of one or more eyelets per haptic and enlargement of haptic ends aid to minimise rate of slippage and IOL dislocation [134]. Haptic visibility through the sclera or conjunctiva has been described in 50% of cases after glued IOL technique by Kumar et al. [118]. However, most of these are considered as not clinically significant in the absence of conjunctival rubbing of the haptic tip. Real erosion has been previously described by the same author in 2–4% of cases [81].
Endophthalmitis
Endophthalmitis is a complication related to any intraocular surgery. Nonetheless, there is a higher risk in patients undergoing SFIOL implantation if there is inadvertent wound leak and suture or haptic erosion [134]. Kannan et al. [141] found only four cases of acute postoperative endophthalmitis in 3541 eyes after sutureless, glueless, flapless SFIOL implantation combined with PPV (0.112%). Of these, only one case was confirmed by a positive culture of Pseudomonas aeruginosa. Mean time to presentation was 10.25 + 9.6 days. Regarding management, two cases resolved after single intravitreal injection of antibiotics, and two required vitrectomy.
Unfortunately, suture erosion or haptic extrusion can occur even after a well performed technique and they should be addressed as soon as possible to preserve globe integrity and to avoid passage of microorganisms through the suture or haptic tracts. This can be achieved either by suture repositioning, removal or coverage with donor grafts [139]. Patients with pre-existing extensive scarring or scleroconjuntival thinning may not be suitable candidates for scleral-fixation IOL due to a higher risk of significant late exposure. Additional risk factors for endophthalmitis include contaminated glue or infusions, large scleral pockets or tunnels which enable bacterial growth, and vitreous wicks in non-sealing wounds [141].
Lens tilt and decentration
The risk of major IOL tilt and subluxation has been a concern after SFIOL surgery and postoperative outcomes should match those accomplished after uneventful cataract extraction, where the mean optic microtilt of in-the-bag IOL has been reported to lie between 1.13 and 1.52 degrees [142]. It has been demonstrated through Purkinje imaging analysis that an optic tilt angle of less than 5 degrees does not have an impact in quality of vision or induced refractive error. With newer SFIOL techniques achieving better and more numerous points of fixation, significant tilt and dislocation becomes less likely [143].
Primary attempts to perform IOL scleral fixation with two anchoring points increased the risk of significant lens tilt of >5 degrees. Durak et al. [144] reported a mean tilt of 6.09 + 3.80 degrees in 53 patients who underwent transscleral sutured PCIOL; decentration >1 mm and optic tilt >10 degrees was present in 16.7% of cases. Analysis with anterior segment OCT scans following glued SFIOL has shown a mean optic microtilt of 0.8 + 1.7 degrees on the vertical axis and of 0.4 + 1.2 degrees on the horizontal axis in 20% of cases, with minimal decentration [118]. After sutureless flanged technique described by Yamane et al. [1] mean IOL tilt was reported to be 3.4 + 2.5 degrees.
Since It is not possible to visualise the exact point of trocar or needle insertion, we rely on important anatomical landmarks such as the posterior surgical limbus. With the advent of new imaging devices, there has been a more detailed definition of the surface and shape of the ciliary sulcus in vivo. Sugiura et al. [145] demonstrated, through ultrasound biomcroscopy and endoscopic surgery, that the limits of the pars plicata are located at ~2.4 mm from the posterior surgical limbus when viewed from an outside perspective. Thus, they recommend a needle emergence point of 2.5 mm for ab interno ciliary sulcus fixation, and a needle insertion point at 3 mm for ab externo pars plana fixation technique. Haptic fixation points <2.4 mm increase the risk of bleeding and more pronounced optic tilt, since the haptics would be fixated over the ciliary processes. Fixation further than 3 mm from posterior surgical limbus increases the distance between two anchoring points, inducing unnecessary stretching and increased total IOL length. Additionally, ensuring a symmetrical haptic placement at 180 degrees from each other will help reduce suture-related optic tilt and better long-term IOL stability.
Retinal detachment
The rate of retinal detachment after SFIOL sutured has been reported to be between 1 and 8% [35, 51]. However, this rate could be higher in predisposed patients. Dimopoulos et al. [44] found a retinal detachment rate of 15% in a series of 62 eyes undergoing knotless sutured technique; half of these patients had history or trauma and previous retinal detachment repair.
Lee et al. [146] reported a higher but non-significant difference in development of retinal detachment after primary vs secondary transscleral sutured PCIOL implantation (8.1% vs 1.7%, respectively). The former group comprised patients with ruptured posterior capsule, suggesting an important role of vitreous traction in the pathogenesis of the detachment. Cases that developed intraocular haemorrhage were significantly correlated with a higher incidence of retinal detachment and proliferative vitreoretinopathy. Also, most tears were located near the haptic fixation points. In order to reduce rates of retinal detachment, it is recommended to perform vitrectomy with a thorough vitreous base shave especially near the sclerotomy sites, avoiding hypotony, controlling major sources of intraoperative bleeding, and treating high risk retinal pathology related to myopia or trauma [55].
Conclusion
It is clear that techniques of lens implantation in the absence of zonular or capsular support will be necessary for the foreseeable future for a range of ophthalmic pathologies and surgical complications. The multiple techniques available and the absence of a consensus on the optimal surgical approach is evidence that further work on developing techniques is required. Existing published studies have obvious limitations—particularly the absence on controlled long-term follow-up and the comparison of differing patient groups. There remains a need for detailed prospective studies of long-term outcomes (5–10 years)—the experience at Moorfields where the rate of suture breakage only became apparent with ongoing long-term analysis highlights this [51]. Future studies would ideally analyse corneal endothelial cell counts in addition to other reported outcomes. Organising such studies is resource intensive and problematic over prolonged periods. Randomised trials comparing IOL placement and techniques could potentiality provide valuable data on surgical options. However, they have the potential for bias as participating surgeons may be more skilled in (and have a preference for) one surgical approach over another. These cases often have multiple ophthalmic co-morbidities and the most appropriate technique may differ accordingly, making comparisons of surgical approach problematic. This is particularly true in the area of lens implantation without zonular or capsular support where surgical techniques are relatively complex and cases infrequent at present. Therefore, surgeon choice remains important—differing surgical skills are required for the varied techniques described above and individual surgeons will select their surgical approach accordingly.
References
Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged Intrascleral Intraocular Lens Fixation with Double-Needle Technique. In: Ophthalmology. Vol 124. Elsevier Inc.; 2017. p. 1136–42. https://doi.org/10.1016/j.ophtha.2017.03.036.
Stem MS, Todorich B, Woodward MA, Hsu J, Wolfe JD. Scleral-Fixated Intraocular Lenses. J Vitreoretin Dis. 2017;1:144–52. https://doi.org/10.1177/2474126417690650.
Negretti GS, Lai M, Petrou P, Walker R, Charteris D. Anterior chamber lens implantation in vitrectomised eyes. Eye. 2018;32:597–601. https://doi.org/10.1038/eye.2017.261.
Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL. Intraocular lens implantation in the absence of capsular support: A report by the American Academy of Ophthalmology. Ophthalmology. 2003;110:840–59. https://doi.org/10.1016/S0161-6420(02)02000-6.
Holt DG, Young J, Stagg B, Ambati BK. Anterior chamber intraocular lens, sutured posterior chamber intraocular lens, or glued intraocular lens: Where do we stand? Curr Opin Ophthalmol. 2012;23:62–67. https://doi.org/10.1097/ICU.0b013e32834cd5e5.
Toro MD, Longo A, Avitabile T, Nowomiejska K, Gagliano C, Tripodi S, et al. Five-year follow-up of secondary iris-claw intraocular lens implantation for the treatment of aphakia: Anterior chamber versus retropupillary implantation. PLoS One. 2019;14. https://doi.org/10.1371/journal.pone.0214140.
Choi EY, Lee CH, Kang HG, Han JY, Byeon SH, Kim SS, et al. Long-term surgical outcomes of primary retropupillary iris claw intraocular lens implantation for the treatment of intraocular lens dislocation. Sci Rep. Published online 2021. https://doi.org/10.1038/s41598-020-80292-3.
Gonnermann J, Klamann MKJ, Maier AK, Rjasanow J, Joussen AM, Bertelmann E, et al. Visual outcome and complications after posterior iris-claw aphakic intraocular lens implantation. J Cataract Refract Surg. Published online 2012. https://doi.org/10.1016/j.jcrs.2012.07.035.
Smiddy WE, Sawusch MR, O’Brien TP, Scott DR, Huang SS. Implantation of scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1990;16:691–6. https://doi.org/10.1016/S0886-3350(13)81007-3.
Long C, Wei Y, Yuan Z, Zhang Z, Lin X, Liu B. Modified technique for transscleral fixation of posterior chamber intraocular lenses. BMC Ophthalmol. 2015;15. https://doi.org/10.1186/s12886-015-0118-8.
Kumar DA, Agarwal A, Ali WRK. Uveitis and deficient lens capsules: effect of glued intraocular lens on the visual outcome and the reactivation of inflammation. Indian J Ophthalmol. 2019;67:1610–6. https://doi.org/10.4103/ijo.IJO_20_19.
Todorich B, Thanos A, Yonekawa Y, Thomas BJ, Abbey AM, Faia LJ. Transconjunctival sutureless intrascleral fixation of secondary intraocular lenses in patients with uveitis. Ocul Immunol Inflamm. 2018;26:456–60. https://doi.org/10.1080/09273948.2016.1231328.
Zeh WG, Price FW. Iris fixation of posterior chamber intraocular lenses. J Cataract Refract Surg. 2000;26:1028–34. https://doi.org/10.1016/S0886-3350(00)00322-9.
Welsh RC. Defects of vision through aphakic spectacle lenses. Br J Ophthalmol. 1967. https://doi.org/10.1136/bjo.51.5.306.
Dabezies OH. Defects of Vision Through Aphakic Spectacle Lenses. Ophthalmology. https://doi.org/10.1016/S0161-6420(79)35503-8.
Durham DG, Kuhwald EP, Kuhwald EP. Extended wear of hydrophilic lenses in aphakia—an alternative to lens implantation. Del Med J. 1979.
He T, You C, Chen S, Meng X, Liu Y, Yan H. Secondary sulcus-fixed foldable IOL implantation with 25-G infusion in patients with previous PPV after open-globe injury. Eur J Ophthalmol. 2017;27:786–90. https://doi.org/10.5301/ejo.5000963.
Cho BJ, Yu HG. Surgical outcomes according to vitreous management after scleral fixation of posterior chamber intraocular lenses. Retina. 2014;34:1977–84. https://doi.org/10.1097/IAE.0000000000000168.
Can E, Başaran R, Gül A, Birinci H. Scleral fixation of one piece intraocular lens by injector implantation. Indian J Ophthalmol. 2014;62:857–60. https://doi.org/10.4103/0301-4738.141042.
Rishi P, Rishi E, Maitray A. Surgical refixation of posteriorly dislocated intraocular lens with scleral-tuck technique. Indian J Ophthalmol. 2017;65:365–70. https://doi.org/10.4103/ijo.IJO_960_16.
Oh SY, Lee SJ, Park JM. Comparision of surgical outcomes of intraocular lens refixation and intraocular lens exchange with perfluorocarbon liquid and fibrin glue-assisted sutureless scleral fixation. Eye. 2015. https://doi.org/10.1038/eye.2015.22.
Eum SJ, Kim MJ, Kim HK A. Comparison of Clinical Outcomes of Dislocated Intraocular Lens Fixation between In Situ Refixation and Conventional Exchange Technique Combined with Vitrectomy. J Ophthalmol. 2016. https://doi.org/10.1155/2016/5942687.
Kristianslund O, Østern AE, Drolsum L. Astigmatism and refractive outcome after late in-the-bag intraocular lens dislocation surgery: A randomized clinical trial. Investig Ophthalmol Vis Sci. 2017. https://doi.org/10.1167/iovs.17-22723.
Shin YI, Park UC. Surgical Outcome of Refixation versus Exchange of Dislocated Intraocular Lens: A Retrospective Cohort Study. J Clin Med. 2020. https://doi.org/10.3390/jcm9123868.
Dalby M, Kristianslund O, Drolsum L. Long-Term Outcomes after Surgery for Late In-The-Bag Intraocular Lens Dislocation: A Randomized Clinical Trial. Am J Ophthalmol. 2019. https://doi.org/10.1016/j.ajo.2019.05.030.
Cheung CSY, VanderVeen DK. Intraocular Lens Techniques in Pediatric Eyes with Insufficient Capsular Support: Complications and Outcomes. Semin Ophthalmol. 2019. https://doi.org/10.1080/08820538.2019.1620809.
Lambert SR, Buckley EG, Drews-Botsch C, DuBois L, Hartmann EE, Lynn MJ, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: Grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010. https://doi.org/10.1001/archophthalmol.2010.101.
Sen P, S. VK, Bhende P, Rishi P, Rishi E, Rao C, et al. Surgical outcomes and complications of sutured scleral fixated intraocular lenses in pediatric eyes. Can J Ophthalmol. 2018. https://doi.org/10.1016/j.jcjo.2017.07.015.
Sen P, Attiku Y, Bhende P, Rishi E, Ratra D, Sreelakshmi K. Outcome of sutured scleral fixated intraocular lens in Marfan syndrome in pediatric eyes. Int Ophthalmol. 2020. https://doi.org/10.1007/s10792-020-01322-7.
Kumar DA, Agarwal A, Prakash D, Prakash G, Jacob S, Agarwal A. Glued intrascleral fixation of posterior chamber intraocular lens in children. Am J Ophthalmol. 2012. https://doi.org/10.1016/j.ajo.2011.09.027.
Nb K, Kohli P, Pangtey BPS, Ramasamy K. Evaluation of Sutureless, Glueless, Flapless, Intrascleral Fixated Posterior Chamber Intraocular Lens in Children with Ectopia Lentis. J Ophthalmol. 2018. https://doi.org/10.1155/2018/3212740.
Solomon K, Gussler JR, Gussler C, Van Meter WS. Incidence and management of complications of transsclerally sutured posterior chamber lenses. J Cataract Refract Surg. 1993;19:488–93. https://doi.org/10.1016/S0886-3350(13)80612-8.
Fass ON, Herman WK. Four-point suture scleral fixation of a hydrophilic acrylic IOL in aphakic eyes with insufficient capsule support. J Cataract Refract Surg. 2010;36:991–6. https://doi.org/10.1016/j.jcrs.2009.12.043.
Szurman P, Petermeier K, Aisenbrey S, Spitzer MS, Jaissle GB. Z-suture: A new knotless technique for transscleral suture fixation of intraocular implants. Br J Ophthalmol. 2010;94:167–9. https://doi.org/10.1136/bjo.2009.162180.
Luk ASW, Young AL, Cheng LL. Long-term outcome of scleral-fixated intraocular lens implantation. Br J Ophthalmol. 2013;97:1308–11. https://doi.org/10.1136/bjophthalmol-2013-303625.
Agrawal S, Singh V, Gupta S, Misra N, Srivastava R. Transscleral fixation of closed loop haptic acrylic posterior chamber intraocular lens in aphakic nonvitrectomized eyes. Indian J Ophthalmol. 2015. https://doi.org/10.4103/0301-4738.169797.
Yang JM, Yoon KC, Ji YS. Transscleral fixation of single-piece foldable acrylic lens with eyelets at the optic-haptic junction. Can J Ophthalmol. 2015. https://doi.org/10.1016/j.jcjo.2015.07.006.
Khan MA, Gupta OP, Smith RG, Ayres BD, Raber IM, Bailey RS, et al. Scleral fixation of intraocular lenses using Gore-Tex suture: clinical outcomes and safety profile. Br J Ophthalmol. 2016;100:638–43. https://doi.org/10.1136/bjophthalmol-2015-306839.
Khan MA, Rahimy E, Gupta OP, Hsu J. Combined 27-gauge pars plana vitrectomy and scleral fixation of an akreos AO60 intraocular lens using Gore-Tex suture. Retina. 2016;36:1602–4. https://doi.org/10.1097/IAE.0000000000001147.
Terveen DC, Fram NR, Ayres B, Berdahl JP. Small-incision 4-point scleral suture fixation of a foldable hydrophilic acrylic intraocular lens in the absence of capsule support. J Cataract Refract Surg. 2016. https://doi.org/10.1016/j.jcrs.2015.10.068.
Dinç E, Vatansever M, Dursun Ö, Markirt HG, Aydin B, Yilmaz A, et al. Scleral fixated intraocular lens implantation with a modified Z-suture technique. Int Ophthalmol 2018. https://doi.org/10.1007/s10792-017-0735-8.
Yeung L, Wang NK, Wu WC, Chen KJ. Combined 23-gauge transconjunctival vitrectomy and scleral fixation of intraocular lens without conjunctival dissection in managing lens complications. BMC Ophthalmol Published online. 2018. https://doi.org/10.1186/s12886-018-0776-4.
Kershner RM. Simple method of transscleral fixation of a posterior chamber intraocular lens in the absence of the lens capsule. J Refract Corneal Surg. 1994. https://doi.org/10.3928/1081-597X-19941101-10.
Dimopoulos S, Dimopoulos V, Blumenstock G, Trevino-Rodriguez H, Bartz-Schmidt KU, Spitzer MS, et al. Long-term outcome of scleral-fixated posterior chamber intraocular lens implantation with the knotless Z-suture technique. J Cataract Refract Surg. 2018. https://doi.org/10.1016/j.jcrs.2017.11.009.
Goel N. Clinical outcomes of combined pars plana vitrectomy and trans-scleral 4-point suture fixation of a foldable intraocular lens. Eye. 2018. https://doi.org/10.1038/s41433-018-0018-2.
Wasiluk E, Krasnicki P, Dmuchowska DA, Proniewska-Skrętek E, Mariak Z. The implantation of the scleral-fixated posterior chamber intraocular lens with 9/0 polypropylene sutures—Long-term visual outcomes and complications. Adv Med Sci. 2019;64:100–3. https://doi.org/10.1016/j.advms.2018.08.005.
Zhao H, Wang W, Hu Z, Chen B. Long-term outcome of scleral-fixated intraocular lens implantation without conjunctival peritomies and sclerotomy in ocular trauma patients. BMC Ophthalmol. 2019;19. https://doi.org/10.1186/s12886-019-1172-4.
Kaynak S, Ozbek Z, Pasa E, Oner FH, Cingil G. Transscleral fixation of foldable intraocular lenses. J Cataract Refract Surg. 2004. https://doi.org/10.1016/j.jcrs.2003.09.050.
Baykara M, Avci R. Prevention of suture knot exposure in posterior chamber intraocular lens implantation by 4-point scleral fixation technique. Ophthalmic Surg Lasers Imaging. 2004. https://doi.org/10.3928/1542-8877-20040901-06.
Young AL, Leung GYS, Cheng LL, Lam DSC. A modified technique of scleral fixated intraocular lenses for aphakic correction. Eye. 2005;19:19–22. https://doi.org/10.1038/sj.eye.6701412.
Vote BJ, Tranos P, Bunce C, Charteris DG, Da Cruz L. Long-term outcome of combined pars plana vitrectomy and scleral fixated sutured posterior chamber intraocular lens implantation. Am J Ophthalmol. 2006;141. https://doi.org/10.1016/j.ajo.2005.09.012.
Bading G, Hillenkamp J, Sachs HG, Gabel VP, Framme C. Long-term Safety and Functional Outcome of Combined Pars Plana Vitrectomy and Scleral-Fixated Sutured Posterior Chamber Lens Implantation. Am J Ophthalmol. 2007;144. https://doi.org/10.1016/j.ajo.2007.05.014.
Krause L, Bechrakis NE, Heimann H, Salditt S, Foerster MH. Implantation of scleral fixated sutured posterior chamber lenses: a retrospective analysis of 119 cases. Int Ophthalmol. 2009. https://doi.org/10.1007/s10792-008-9217-3.
Kim SJ, Lee SJ, Park CH, Jung GY, Park SH. Long-term stability and visual outcomes of a single-piece, foldable, acrylic intraocular lens for scleral fixation. Retina. 2009. https://doi.org/10.1097/IAE.0b013e318188c7fc.
Por YM, Lavin MJ. Techniques of intraocular lens suspension in the absence of capsular/zonular support. Surv Ophthalmol. 2005;50:429–62. https://doi.org/10.1016/j.survophthal.2005.06.010.
Malbran ES, Malbran E, Negri I. Lens guide suture for transport and fixation in secondary IOL implantation after intracapsular extraction. Int Ophthalmol. 1986;9:151–60. https://doi.org/10.1007/BF00159844.
Lewis JS. Ab externo sulcus fixation. Ophthalmic Surg. 1991;22:692–5. https://doi.org/10.3928/1542-8877-19911101-14.
Hadayer A, Puri S, Fassbender Adeniran J, Wang W, Kaplan HJ. Minimally Invasive Ab Interno Four-Point Scleral Fixation of Intraocular Lens. Retina. 2019. https://doi.org/10.1097/IAE.0000000000002138.
Lewis JS. Sulcus Fixation without Flaps. Ophthalmology. 1993;100:1346–50. https://doi.org/10.1016/S0161-6420(93)31477-6.
Hoffman RS, Fine IH, Packer M. Scleral fixation without conjunctival dissection. J Cataract Refract Surg. 2006;32:1907–12. https://doi.org/10.1016/j.jcrs.2006.05.029.
Almashad GY, Abdelrahman AM, Khattab HA, Samir A. Four-point scleral fixation of posterior chamber intraocular lenses without scleral flaps. Br J Ophthalmol. 2010;94:693–5. https://doi.org/10.1136/bjo.2009.161349.
Ni S, Wang W, Chen X, Wu X, He S, Ma Y, et al. Clinical observation of a novel technique: Transscleral suture fixation of a foldable 3-looped haptics one-piece posterior chamber intraocular lens implantation through scleral pockets with intact conjunctiva. BMC Ophthalmol. 2019. https://doi.org/10.1186/s12886-019-1113-2.
Fass ON, Herman WK. Sutured intraocular lens placement in aphakic post-vitrectomy eyes via small-incision surgery. J Cataract Refract Surg. 2009;35:1492–7. https://doi.org/10.1016/j.jcrs.2009.04.034.
Kalevar A, Dollin M, Gupta RR. Opacification of Scleral-Sutured Akreos Ao60 Intraocular Lens After Vitrectomy with Gas Tamponade: Case Series. Retin Cases Brief Rep. 2020;14:174–7. https://doi.org/10.1097/ICB.0000000000000634.
Gregori NZ, Echegaray JJ, Flynn HW. Opacification of Akreos Hydrophilic Acrylic Lens After Retinal Detachment Repair with Silicone Oil Tamponade: A Case Report. Ophthalmol Ther. 2019;8:341–5. https://doi.org/10.1007/s40123-019-0183-9.
Salvador L, Mirone S, Bianchini R, Regesta T, Patelli F, Minniti G, et al. A 20-year experience with mitral valve repair with artificial chordae in 608 patients. J Thorac Cardiovasc Surg. 2008;135. https://doi.org/10.1016/j.jtcvs.2007.12.026.
Khan MA, Gerstenblith AT, Dollin ML, Gupta OP, Spirn MJ. Scleral fixation of posterior chamber intraocular lenses using gore-tex suture with concurrent 23-gauge pars plana vitrectomy. Retina. 2014;34:1477–80. https://doi.org/10.1097/IAE.0000000000000233.
Nadal J, Kudsieh B, Casaroli-Marano RP. Scleral fixation of posteriorly dislocated intraocular lenses by 23-gauge vitrectomy without anterior segment approach. J Ophthalmol. 2015;2015. https://doi.org/10.1155/2015/391619.
Johnston RL, Charteris DG, Horgan SE, Cooling RJ. Combined pars plana vitrectomy and sutured posterior chamber implant. Arch Ophthalmol. 2000;118:905–10.
Yang YF, Bunce C, Dart JKG, Johnston RL, Charteris DG. Scleral-fixated posterior chamber intraocular lenses in nonvitrectomised eyes. Eye. 2006;20:64–70. https://doi.org/10.1038/sj.eye.6701804.
Gabor SGB, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007;33:1851–4. https://doi.org/10.1016/j.jcrs.2007.07.013.
Scharioth GB, Prasad S, Georgalas I, Tataru C, Pavlidis M. Intermediate results of sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2010;36:254–9. https://doi.org/10.1016/j.jcrs.2009.09.024.
Prenner JL, Feiner L, Wheatley HM, Connors D. A novel approach for posterior chamber intraocular lens placement or rescue via a sutureless scleral fixation technique. Retina. 2012;32:853–5. https://doi.org/10.1097/IAE.0b013e3182479b61.
Wilgucki JD, Wheatley HM, Feiner L, Ferrone MV, Prenner JL. One-year outcomes of eyes treated with a sutureless scleral fixation technique for intraocular lens placement or rescue. Retina. 2015;35:1036–40. https://doi.org/10.1097/IAE.0000000000000431.
Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinivasan S. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg. 2008;34:1433–8. https://doi.org/10.1016/j.jcrs.2008.04.040.
Kumar DA, Agarwal A, Prakash G, Jacob S, Saravanan Y, Agarwal A. Glued posterior chamber IOL in eyes with deficient capsular support: A retrospective analysis of 1-year post-operative outcomes. Eye. 2010;24:1143–8. https://doi.org/10.1038/eye.2010.10.
Agarwal A, Jacob S, Kumar DA, Agarwal A, Narasimhan S, Agarwal A. Handshake technique for glued intrascleral haptic fixation of a posterior chamber intraocular lens. J Cataract Refract Surg. 2013;39:317–22. https://doi.org/10.1016/j.jcrs.2013.01.019.
Beiko G, Steinert R. Modification of externalized haptic support of glued intraocular lens technique. J Cataract Refract Surg. 2013;39:323–5. https://doi.org/10.1016/j.jcrs.2013.01.017.
McKee Y, Price FW, Feng MT, Price MO. Implementation of the posterior chamber intraocular lens intrascleral haptic fixation technique (glued intraocular lens) in a United States practice: Outcomes and insights. J Cataract Refract Surg. 2014;40:2099–105. https://doi.org/10.1016/j.jcrs.2014.04.027.
Kumar DA, Agarwal A, Agarwal A, Prakash G, Jacob S. Glued intraocular lens implantation for eyes with defective capsules: A retrospective analysis of anatomical and functional outcome. Saudi J Ophthalmol. 2011;25:245–54. https://doi.org/10.1016/j.sjopt.2011.04.001.
Kumar DA, Agarwal A. Glued intraocular lens: A major review on surgical technique and results. Curr Opin Ophthalmol. 2013;24:21–29. https://doi.org/10.1097/ICU.0b013e32835a939f.
Yamane S, Inoue M, Arakawa A, Kadonosono K. Sutureless 27-gauge needle-guided intrascleral intraocular lens implantation with lamellar scleral dissection. In: Ophthalmology. Vol 121. Ophthalmology; 2014:61–66. https://doi.org/10.1016/j.ophtha.2013.08.043.
Yavuzer K, Evcimen Y. Sutureless transconjunctival intrascleral intraocular lens fixation: the modified Yamane technique. Arq Bras Oftalmol. 2019;82:389–93. https://doi.org/10.5935/0004-2749.20190072.
Gelman RA, Garg S. Novel yamane technique modification for haptic exposure after glued intrascleral haptic fixation. Am J Ophthalmol Case Rep. 2019;14:101–4. https://doi.org/10.1016/j.ajoc.2019.03.009.
Todorich B, Thanos A, Woodward MA, Wolfe JD. Sutureless intrascleral fixation of secondary intraocular lens using 27-gauge vitrectomy system. Ophthalmic Surg Lasers Imaging Retin. 2016;47:376–9. https://doi.org/10.3928/23258160-20160324-14.
Todorich B, Stem MS, Kooragayala K, Thanos A, Faia LJ, Williams GA, et al. Structural analysis and comprehensive surgical outcomes of the sutureless intrascleral fixation of secondary intraocular lenses in human eyes. Retina. 2017;38:S31–S40. https://doi.org/10.1097/IAE.0000000000001941.
Stem MS, Wa CA, Todorich B, Woodward MA, Walsh MK, Wolfe JD. 27-gauge sutureless intrascleral fixation of intraocular lenses with haptic flanging: short-term clinical outcomes and a disinsertion force study. Retina. 2019;39:2149–54. https://doi.org/10.1097/IAE.0000000000002268.
Barca F, Caporossi T, de Angelis L, Giansanti F, Savastano A, Leo LD, et al. “Sutureless scleral fixation using Carlevale lens. J Cataract Refract Surg. 2020:1. https://doi.org/10.1097/j.jcrs.0000000000000135.
Veronese C, Maiolo C, Armstrong GW, Primavera L, Torrazza C, Mora LD, et al. New surgical approach for sutureless scleral fixation. Eur J Ophthalmol. 2020;30. https://doi.org/10.1177/1120672120902020.
Narang P, Narang S. Glue-assisted intrascleral fixation of posterior chamber intraocular lens. Indian J Ophthalmol. 2013. https://doi.org/10.4103/0301-4738.112160.
Kawaji T, Sato T, Tanihara H. Sutureless intrascleral intraocular lens fixation with lamellar dissection of scleral tunnel. Clin Ophthalmol. 2016. https://doi.org/10.2147/OPTH.S101515.
Agarwal L, Agarwal N, Gurung RL, Chaubey R, Jha BK, Chaudhary BP. Visual outcome and early complications of sutureless and glueless scleral fixated intraocular lens. Nepal J Ophthalmol. 2016. https://doi.org/10.3126/nepjoph.v8i1.16155.
Kelkar AS, Fogla R, Kelkar J, Kothari AA, Mehta H, Amoaku W. Sutureless 27-gauge needle-Assisted transconjunctival intrascleral intraocular lens fixation: Initial experience. Indian J Ophthalmol. 2017. https://doi.org/10.4103/ijo.IJO_659_17.
Kansal V, Onasanya O, Colleaux K, Rawlings N. Outcomes of Using Sutureless, Scleral-Fixated Posterior Chamber Intraocular Lenses. Semin Ophthalmol. 2019. https://doi.org/10.1080/08820538.2019.1652761.
Walia S, Kashyap S, Bhaisare V, Rawat P, Kori N. Novel technique of sutureless glueless scleral fixated intraocular lens (SFIOL). Indian J Ophthalmol. 2019. https://doi.org/10.4103/ijo.IJO_447_18.
Boral SK, Agarwal D. A Simple Modified Way of Glueless, Sutureless Scleral Fixation of an IOL: A Retrospective Case Series. Am J Ophthalmol. 2020. https://doi.org/10.1016/j.ajo.2020.03.021.
Postorino M, Meduri A, Inferrera L, Tumminello G, Rechichi M, Caparello O, et al. Scleral pockets for an innovative technique of intrascleral fixation of intraocular lens. Eur J Ophthalmol. 2020. https://doi.org/10.1177/1120672119866018.
Madhivanan N, Sengupta S, Sindal M, Nivean PD, Kumar MA, Ariga M. Comparative analysis of retropupillary iris claw versus scleral-fixated intraocular lens in the management of post-cataract aphakia. Indian J Ophthalmol. 2019. https://doi.org/10.4103/ijo.IJO_326_18.
Kelkar AS, Kelkar JA, Kothari AA, Kelkar SB. Comparison of flanged intrascleral intraocular lens fixation versus iris claw intraocular lens fixation: A retrospective study. Indian J Ophthalmol. 2019. https://doi.org/10.4103/ijo.IJO_300_19.
Vounotrypidis E, Schuster I, Mackert MJ, Kook D, Priglinger S, Wolf A. Secondary intraocular lens implantation: a large retrospective analysis. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:125–34. https://doi.org/10.1007/s00417-018-4178-3.
Ganekal S, Venkataratnam S, Dorairaj S, Jhanji V. Comparative evaluation of suture-assisted and fibrin glue—Assisted scleral fixated intraocular lens implantation. J Refract Surg. 2012;28:249–52. https://doi.org/10.3928/1081597X-20120221-01.
Haszcz D, Nowomiejska K, Oleszczuk A, Forlini C, Forlini M, Moneta-Wielgos J. et al. Visual outcomes of posterior chamber intraocular lens intrascleral fixation in the setting of postoperative and posttraumatic aphakia. BMC Ophthalmol. 2016;16. https://doi.org/10.1186/s12886-016-0228-y.
Sindal MD, Nakhwa CP, Sengupta S. Comparison of sutured versus sutureless scleral-fixated intraocular lenses. J Cataract Refract Surg. 2016;42:27–34. https://doi.org/10.1016/j.jcrs.2015.09.019.
Erdogan G, Unlu C, Gunay BO, Kardes E, Ergin A. Implantation of foldable posterior chamber intraocular lens in aphakic vitrectomized eyes without capsular support. Arq Bras Oftalmol. 2016.
Sinha R, Bansal M, Sharma N, Dada T, Tandon R, Titiyal JS. Transscleral suture-fixated versus intrascleral haptic-fixated intraocular lens: A comparative study. Eye Contact Lens. 2017. https://doi.org/10.1097/ICL.0000000000000287.
Balakrishnan D, Mukundaprasad V, Jalali S, Pappuru RR. A Comparative Study on Surgical Outcomes of Glued Intraocular Lens and Sutured Scleral Fixated Intraocular Lens Implantation. Semin Ophthalmol. 2018. https://doi.org/10.1080/08820538.2017.1346132.
Li X, Ni S, Li S, Zheng Q, Wu J, Liang G, et al. Comparison of Three Intraocular Lens Implantation Procedures for Aphakic Eyes With Insufficient Capsular Support: A Network Meta-analysis. Am J Ophthalmol. 2018. https://doi.org/10.1016/j.ajo.2018.04.023.
Evereklioglu C, Er H, Bekir NA, Borazan M, Zorlu F. Comparison of secondary implantation of flexible open-loop anterior chamber and scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 2003;29:301–8. https://doi.org/10.1016/S0886-3350(02)01526-2.
Cho YW, Yoo WS, Chung I, Seo SW, Yoo JM, Kim SJ. Short-term Clinical Outcomes after Transscleral Fixation Using the Intrascleral Pocket Technique: A Retrospective Cohort Study Analysis. Korean J Ophthalmol. 2016. https://doi.org/10.3341/kjo.2016.30.2.108.
Chantarasorn Y, Techalertsuwan S, Siripanthong P, Tamerug A. Reinforced scleral fixation of foldable intraocular lens by double sutures: comparison with intrascleral intraocular lens fixation. Jpn J Ophthalmol. 2018. https://doi.org/10.1007/s10384-018-0579-4.
Dadeya S, Kamlesh, Sodhi PK. Secondary intraocular lens (IOL) implantation: Anterior chamber versus scleral fixation long-term comparative evaluation. Eur J Ophthalmol. 2003. https://doi.org/10.1177/112067210301300706.
Kwong YYY, Yuen HKL, Lam RF, Lee VYW, Rao SK, Lam DSC. Comparison of Outcomes of Primary Scleral-Fixated versus Primary Anterior Chamber Intraocular Lens Implantation in Complicated Cataract Surgeries. Ophthalmology. 2007. https://doi.org/10.1016/j.ophtha.2005.11.024.
Chan TCY, Lam JKM, Jhanji V, Li EYM. Comparison of outcomes of primary anterior chamber versus secondary scleral-fixated intraocular lens implantation in complicated cataract surgeries. Am J Ophthalmol. 2015. https://doi.org/10.1016/j.ajo.2014.10.016.
Khan MA, Gupta OP, Pendi K, Chiang A, Vander J, Regillo CD, et al. Pars plana vitrectomy with anterior chamber versus gore-tex sutured posterior chamber intraocular lens placement: long-term outcomes. Retina. 2019;39:860–6. https://doi.org/10.1097/IAE.0000000000002042.
Zheng D, Wan P, Liang J, Song T, Liu Y. Comparison of clinical outcomes between iris-fixated anterior chamber intraocular lenses and scleral-fixated posterior chamber intraocular lenses in Marfan syndrome with lens subluxation. Clin Exp Ophthalmol. 2012. https://doi.org/10.1111/j.1442-9071.2011.02612.x.
Kim KH, Kim WS. Comparison of clinical outcomes of iris fixation and scleral fixation as treatment for intraocular lens dislocation. Am J Ophthalmol. 2015;160:463–469. https://doi.org/10.1016/j.ajo.2015.06.010. e1.
Jing W, Guanlu L, Qianyin Z, Shuyi L, Fengying H, Jian L, et al. Iris-claw intraocular lens and scleral-fixated posterior chamber intraocular lens implantations in correcting aphakia: A meta-analysis. Investig Ophthalmol Vis Sci. 2017. https://doi.org/10.1167/iovs.16-21226.
Kumar DA, Agarwal DA, Dhawan D, Thambusamy V, Sivangnanam S, Venktesh T, et al. Glued intraocular lens in eyes with deficient capsules: A retrospective analysis of long term effects. J Cataract Refract Surg. 2020. https://doi.org/10.1097/j.jcrs.0000000000000416.
Brunin G, Sajjad A, Kim EJ, de Oca IM, Weikert M, Wang L, et al. Secondary intraocular lens implantation: Complication rates, visual acuity, and refractive outcomes. J Cataract Refract Surg. 2017. https://doi.org/10.1016/j.jcrs.2016.12.024.
Hamoudi H, Kofod M, La Cour M. Refractive change after vitrectomy for epiretinal membrane in pseudophakic eyes. Acta Ophthalmol. 2013. https://doi.org/10.1111/j.1755-3768.2012.02574.x.
Byrne S, Ng J, Hildreth A, Danjoux JP, Steel DHW. Refractive change following pseudophakic vitrectomy. BMC Ophthalmol. 2008. https://doi.org/10.1186/1471-2415-8-19.
Lee R, Govindaraju V, Farley ND, Abbey AM, Stem MS, Shields RA, et al. Refractive outcomes after sutureless intrascleral fixation of intraocular lens with pars plana vitrectomy. Retina. 2020. https://doi.org/10.1097/iae.0000000000002916.
Su D, Stephens JD, Obeid A, Borkar D, Storey PP, Khan MA, et al. Refractive outcomes after pars plana vitrectomy and scleral fixated intraocular lens with gore-tex suture. Ophthalmol Retin. 2019;3:548–52. https://doi.org/10.1016/j.oret.2019.02.012.
Kam KW, Chan YFA, Yu M, Ho M, Young AL. Outcomes and complications in scleral-fixated intraocular lens implantations. Int Ophthalmol. 2020;40:2969–77. https://doi.org/10.1007/s10792-020-01480-8.
Rocke JR, McGuinness MB, Atkins WK, Fry LE, Kane JX, Fabinyi DCA, et al. Refractive Outcomes of the Yamane Flanged Intrascleral Haptic Fixation Technique. In: Ophthalmology.; 2020. https://doi.org/10.1016/j.ophtha.2020.03.032.
Haigis W. Pseudophakic correction factors for optical biometry. Graefe’s Arch Clin Exp Ophthalmol. 2001. https://doi.org/10.1007/s004170100323.
Rajan MS, Keilhorn I, Bell JA. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye. 2002. https://doi.org/10.1038/sj.eye.6700157.
Ohr MP, Wisely CE. Refractive outcomes and accuracy of IOL power calculation with the SRK/T formula for sutured, scleral-fixated Akreos AO60 intraocular lenses. Graefe’s Arch Clin Exp Ophthalmol 2020;258:2125–9. https://doi.org/10.1007/s00417-020-04721-9.
Brogan K, Diaper CJM, Rotchford AP. Cataract surgery refractive outcomes: representative standards in a National Health Service setting. Br J Ophthalmol. 2019. https://doi.org/10.1136/bjophthalmol-2018-312209.
Güell JL, Barrera A, Manero F. A review of suturing techniques for posterior chamber lenses. Curr Opin Ophthalmol. 2004. https://doi.org/10.1097/00055735-200402000-00009.
Apple DJ, Brems RN, Park RB, Norman DK, Hansen SO, Tetz MR, et al. Anterior chamber lenses. Part I: Complications and pathology and a review of designs. J Cataract Refract Surg. 1987. https://doi.org/10.1016/S0886-3350(87)80131-1.
Dalby M, Kristianslund O, Østern AE, Falk RS, Drolsum L. Longitudinal corneal endothelial cell loss after corrective surgery for late in-the-bag IOL dislocation: A randomized clinical trial. J Cataract Refract Surg. 2020. https://doi.org/10.1097/j.jcrs.0000000000000213.
Lee VYW, Yuen HKL, Kwok AKH. Comparison of outcomes of primary and secondary implantation of scleral fixated posterior chamber intraocular lens. Br J Ophthalmol. 2003. https://doi.org/10.1136/bjo.87.12.1459.
Davies EC, Pineda R. Complications of Scleral-Fixated Intraocular Lenses. Semin Ophthalmol. 2018. https://doi.org/10.1080/08820538.2017.1353808.
Kjeka O, Bohnstedt J, Meberg K, Seland JH. Implantation of scleral-fixated posterior chamber intraocular lenses in adults. Acta Ophthalmol. 2008. https://doi.org/10.1111/j.1600-0420.2007.01095.x.
Higashide T, Shimizu F, Nishimura A, Sugiyama K. Anterior segment optical coherence tomography findings of reverse pupillary block after scleral-fixated sutured posterior chamber intraocular lens implantation. J Cataract Refract Surg. 2009. https://doi.org/10.1016/j.jcrs.2009.04.030.
Shen JF, Deng S, Hammersmith KM, Kuo AN, Li JY, Weikert MP, et al. Intraocular Lens Implantation in the Absence of Zonular Support: An Outcomes and Safety Update: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020. https://doi.org/10.1016/j.ophtha.2020.03.005.
Price FW, Whitson WE. Suprachoroidal hemorrhage after placement of a scleral-fixated lens. J Cataract Refract Surg. 1990. https://doi.org/10.1016/S0886-3350(13)80811-5.
Price FW, Wellemeyer M. Transscleral fixation of posterior chamber intraocular lenses. J Cataract Refract Surg. 1995;21:567–73. https://doi.org/10.1016/S0886-3350(13)80219-2.
Holland EJ, Djalilian AR, Pederson J. Gonioscopic evaluation of haptic position in transsclerally sutured posterior chamber lenses. Am J Ophthalmol. 1997. https://doi.org/10.1016/S0002-9394(14)70146-1.
Kannan NB, Sen S, Mishra C, Lalitha P, Rameshkumar G, Kumar K, et al. Ten-year trends in the incidence, clinical profile and outcomes of acute-onset endophthalmitis following combined pars plana vitrectomy and sutureless, glueless and flapless scleral fixation of intraocular lenses. Int Ophthalmol. 2021. https://doi.org/10.1007/s10792-021-01715-2.
Kumar DA, Agarwal A, Prakash G, Jacob S, Saravanan Y, Agarwal A. Evaluation of intraocular lens tilt with anterior segment optical coherence tomography. Am J Ophthalmol. 2011. https://doi.org/10.1016/j.ajo.2010.09.013.
Kemer Atik B, Altan C, Agca A, Kirmaci A, Yildirim Y, Genc S, et al. The effect of intraocular lens tilt on visual outcomes in scleral-fixated intraocular lens implantation. Int Ophthalmol. 2020. https://doi.org/10.1007/s10792-019-01233-2.
Durak I, Öner HF, Koçak N, Kaynak S. Tilt and decentration after primary and secondary transsclerally sutured posterior chamber intraocular lens implantation. J Cataract Refract Surg. 2001. https://doi.org/10.1016/S0886-3350(00)00638-6.
Sugiura T, Kaji Y, Tanaka Y. Anatomy of the ciliary sulcus and the optimum site of needle passage for intraocular lens suture fixation in the living eye. J Cataract Refract Surg. 2018. https://doi.org/10.1016/j.jcrs.2018.07.017.
Lee J, Lee JH, Chung H. Factors contributing to retinal detachment after transscleral fixation of posterior chamber intraocular lenses. J Cataract Refract Surg. 1998. https://doi.org/10.1016/S0886-3350(98)80269-1.
Author information
Authors and Affiliations
Contributions
SMS was responsible for designing the review article. SMS, BFS, and RA conducted the literature search and selected the articles pertinent to the review. SMS, BFS, E.C., RA and SNA contributed to writing the different sections of the article. LW and DGC were senior authors responsible for reviewing the final article, making edits and suggesting key changes to improve the accuracy of the article. All authors contributed to the final review of the article before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahid, S.M., Flores-Sánchez, B.C., Chan, E.W. et al. Scleral-fixated intraocular lens implants—evolution of surgical techniques and future developments. Eye 35, 2930–2961 (2021). https://doi.org/10.1038/s41433-021-01571-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01571-5