Abstract
Objectives
To evaluate the effect of locally advanced periocular basal cell carcinoma (POLA-BCC) on health-related quality of life (HRQoL) and the benefit of vismodegib treatment among participants in the Safety Events in Vismodegib (STEVIE) trial between 2011 and 2017.
Methods
The STEVIE trial was conducted in patients with BCC (all anatomic locations) who were treated with vismodegib in 28-day cycles. Patients completed the Skindex-16, a validated questionnaire for the analysis symptoms, emotions, and functioning, at baseline, on day 1 of cycle 2, on day 1 of cycle 7, and at the end-of-study visit. For the present study, data mining techniques were used to construct an ophthalmic database of the STEVIE study. Skindex-16 scores were compared among patients with POLA-BCC between baseline and follow-up and between patients with POLA-BCC and patients with locally advanced BCC on other sites of the head and face (controls).
Results
The cohort included 169 patients with POLA-BCC and 428 patients with non-periocular head BCC. Patients with POLA-BCC had a significantly worse overall functioning score at baseline than controls (p = 0.038) and a lower score specifically in activities of daily living (p = 0.001). At the last follow-up, patients with POLA-BCC showed significant improvement in scores for functioning (100%), symptoms (100%), and emotions (75%) relative to baseline.
Conclusions
Secondary analysis of the results of the STEVIE trial showed that the HRQoL of patients with POLA-BCC is significantly impaired and can be greatly improved with vismodegib treatment.
Similar content being viewed by others
Introduction
Basal cell carcinoma (BCC) is the most frequently diagnosed cancer worldwide. It affects an estimated two million persons in the United States each year and the incidence is gradually increasing [1, 2].
Periocular BCC accounts for 4.4–18% of all BCCs and ~90% of all malignant periocular tumors [1, 3]. In most cases, it is characterized by local spread and a very low tendency to metastasize, and it can be cured by standard surgical interventions. However, a small minority of patients have periocular locally advanced BCC (POLA-BCC) for which surgical treatment is challenging. POLA-BCC may be associated with severe ocular morbidity and loss of vision or even loss of the eye and orbital contents [4, 5].
Health-related quality of life (HRQoL) is evolving as one of the most important treatment goals of modern medicine [6, 7]. Although BCC was originally considered to cause little handicap [8], recent studies reported that disease-related symptoms may affect HRQoL in terms of activities of daily living (ADL), emotional well-being, and social and leisure activities [9]. However, even though HRQoL has been investigated in patients with locally advanced and metastatic BCC [9], there are no studies focusing on POLA-BCC.
Vismodegib (Erivedge®), a hedgehog signaling pathway inhibitor, is emerging as the treatment of choice for locally advanced BCC [10, 11]. The SafeTy Events in VIsmodEgib trial (STEVIE), a single-arm, multicentre, open-label study of the largest group of patients with locally advanced BCC to date, confirmed the safety and efficacy of vismodegib in a setting representative of clinical practice [12]. Further assessment of vismodegib in patients with POLA-BCC in the STEVIE study found that it was tolerable and effective, showing discontinuation of treatment due to adverse events in 23.8%, complete response in 28.7%, and partial response in 38.5% [13]. However, its effect on HRQoL specifically in patients with POLA-BCC has not been evaluated.
Studies have shown that the severe disfigurement associated with periocular conditions can cause high levels of psychosocial distress [14] and that blindness and low vision, which may be a consequence of POLA-BCC, can lead to depression and anxiety [15]. Prompted by these findings, we sought to evaluate the impact of POLA-BCC on HRQoL compared to non-periocular locally advanced head and facial BCC and to determine the benefit of vismodegib on HRQoL in this setting. Data for the study were derived from the STEVIE trial [9, 10].
Methods
STEVIE study
The STEVIE study investigated the safety and efficacy of vismodegib and its effect on HRQoL in patients with BCC treated at 167 sites in 36 countries between July 1, 2011 and June 14, 2017. The study methods and procedures have previously been described in detail [5, 12]. In brief, a single-arm, multicentre, open-label study design was used. Patients received 150 mg of oral vismodegib capsules once a day on a continuous basis in 28-day cycles until progressive disease, toxicity, or withdrawal. HRQoL was evaluated with the Skindex-16, a dermatologic questionnaire with proven reliability and validity [16] covering three domains (Table 1): symptoms (four items), emotions (seven items), and functioning (five items), rated on a scale of 0 (never bothered) to 6 (always bothered). Patients completed the questionnaire before onset of vismodegib treatment (baseline), day 1 of cycle 2, day 1 of cycle 7, and at the end-of-study visit [9, 10]. Scores were aggregated and transformed to yield a maximal total score of 100, with higher scores signifying a greater adverse impact on HRQoL [9, 10].
Secondary analysis of STEVIE data
In the original STEVIE study, findings were analyzed for the cohort as a whole (BCC at any anatomic site) and did not distinguish ocular involvement as a defined tumor site or any other specific ocular consideration. Therefore, for the present study, data mining techniques were used to construct an ophthalmic database of the STEVIE study. Ophthalmic involvement was identified by a natural language processing search for anatomical ophthalmic keywords. All relevant tumor descriptions were evaluated by an experienced orbital surgeon. Scores on the Skindex-16, overall and by domain, were compared among patients with POLA-BCC between baseline and follow-up, and between patients with POLA-BCC and patients with locally advanced BCCs in other areas of the head and face (control group). In the POLA-BCC group, a secondary analysis was performed between patients who completed the treatment and those who terminated their treatment due to adverse events.
Statistical analysis
The relevant demographic and clinical data were compared using descriptive statistics. Analyses of covariance were used to assess the observed differences between groups. Mann–Whitney U test and Wilcoxon signed rank test were applied for paired samples. Chi-square or Fisher’s exact test was used, as appropriate, for nominal variables. Multiple comparisons were adjusted with the Bonferroni correction. All statistical analyses were two-sided, and statistical significance was set at a p value of 0.05. Data were generated with Prism version 7 (GRAPHPAD Software Inc.) and R version 3.4.2 (R Development Core Team 2017).
Results
Patient population
Between July 1, 2011 and June 14, 2017, 1232 patients were enrolled in the STEVIE study. By study completion, 1215 patients had received at least one dose of vismodegib. HRQoL data were available for 169 patients with POLA-BCC and 428 patients with non-POLA-BCC in the head and face. Their clinical characteristics are shown in Table 2. There were no significant between-group differences in any of the parameters evaluated except measurable disease.
Overall Skindex-16 scores: differences between groups
The Skindex-16 results showed that POLA-BCC had a negative effect on HRQoL. The baseline median (range) overall score was 34 (15.5, 53) in the POLA-BCC group and 30 (12, 49) in the control group (p = 0.1327). Both groups demonstrated a meaningful improvement in overall HRQoL score after treatment. The respective end-of-study scores were 9 (0, 29.75, p < 0.001) and 11 (1, 33, p < 0.001), with a 10% greater overall improvement in the POLA-BCC group compared with the control one.
Skindex-16 scores over time: POLA-BCC group
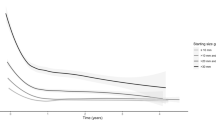
An improvement in HRQoL scores were noted in the POLA-BCC group from baseline to the first day of the second cycle of treatment in all major domains: symptoms [from 4 (0, 9) to 2 (0, 6), p = 0.014], emotions [from 22.5 (11, 33) to 12 (2.75, 24), p < 0.001], and functioning [from 6 (0, 16.5) to 2.5 (0, 12), p < 0.001]. The improvement continued to increase to the end of the study, to 100% for symptoms and functioning [from 6 (0, 17) to 0 (0, 9), p = 0.003 and from 4 (0, 9) to 0 (0, 5), p < 0.001, respectively] and to 75% for emotions [from 22 (11, 33) to 5.5 (0, 19.25, p < 0.001] (Fig. 1).
Forty-seven of the 169 POLA-BCC patients (27.8%) terminated their treatment due to adverse events. No statistically significant differences were observed between them and patients who completed the treatment, in all major domains (Table 3).
Skindex-16 domain scores: differences between groups
At baseline, there were no between-group differences in total score for the symptoms domain (p = 0.979) or in the individual scores for the four items addressing symptoms: skin itching, burning or stinging, hurting, and irritated (Fig. 2).
The total score for the functioning domain was significantly higher in the POLA-BCC group than the control group [6.00 (0.00, 17.00) vs 4.00 (0.00, 13.00), p = 0.038]. ADL accounted for the difference between the groups [1.00 (0.00, 4.00) vs 0.00 (0.00, 3.00), p = 0.001], with no significant differences in the other items addressing functioning: interactions with others (p = 0.864), desire to be with other people (p = 0.221), ability to show affection (p = 0.357), and ability to work or do enjoyable activities (p = 0.115).
In the emotions domain, there was no significant difference in total score between the groups (p = 0.079). Analysis by the individual items revealed that the patients with POLA-BCC were significantly more annoyed about their skin condition than the control patients [3.00 (0.00, 5.00) vs 3.00 (1.00, 6.00), p = 0.049]. There were no significant between-group differences in the other items addressing emotion: worry about the skin condition (p = 0.198), appearance of the skin (p = 0.171), frustration due to the skin condition (p = 0.132), embarrassment (p = 0.083), persistence or recurrence of condition (p = 0.073), and depression (p = 0.306).
Discussion
The primary objective of this secondary subgroup analysis of patients with POLA-BCC in the STEVIE study was to understand the impact of the disease and its treatment (vismodegib) on HRQoL over an extensive follow-up period. We found that POLA-BCC has an adverse impact on HRQoL and this was greatly ameliorated by vismodegib treatment (Fig. 1).
At baseline, patients with POLA-BCC exhibited a high emotional and symptomatic burden and impaired functioning. These findings are in line with previous studies on patients with all types of advanced BCC [17, 18]. Following vismodegib treatment, patients with POLA-BCC showed a significant improvement in all three major domains of the Skindex-16 relative to baseline, with emotional status improving by 75% and symptoms and functional status improving by 100%. In the original STEVIE study, clinically meaningful improvement was defined as a ≥10-point decrease from baseline in the Skindex-16 score, and analysis of the cohort as whole (i.e., BCC at any anatomic location) yielded clinically meaningful vismodegib-associated improvement only in the emotions domain [12]. As the scores for symptoms and functioning were <10 points at baseline, a reduction of more than 10 points using this tool was technically impossible. In contrast to our analysis, Hansson et al. [9] did not report the improvement in Skindex-16 scores as percentages of baseline. Thus, we cannot determine whether the significant improvement in functioning and symptom scores in our study following vismodegib treatment are unique to the periocular subgroup or apply to the entire STEVIE cohort, especially as HRQoL scores responded positively to treatment in both of our groups, nearing normal values.
Our second objective was to compare the HRQoL of patients with POLA-BCC to patients with locally advanced BCC located elsewhere on the head (Fig. 2). The two groups shared many features in the emotions and symptoms domains, although there was an important difference in overall functioning score, which was higher at baseline in the POLA-BCC group, mainly in terms of ADL. This may be explained by the disabling nature of large periocular tumors which can affect functional vision via multiple mechanisms including direct compression, ptosis, ocular surface disturbances, and impaired motility. We expect to gain more insight into the vision-impairing effects of POLA-BCC and its response to treatment from the soon-to-be-published Vismodegib for Orbital and Periocular Basal Cell Carcinoma study [19], which offers a new metric for evaluation of the clinical response to treatment. In addition, studies have shown that disfiguring eye conditions cause high levels of psychological distress [14], which in this instance, may reduce functioning. The disruptive nature of an abnormal eye appearance in social interactions is also well documented. Studies of patients with strabismus reported adverse effects in many aspects of life, including finding a partner, job prospects, and interaction with peers [20]. However, the absence of a between-group difference in our study in baseline scores for emotions and symptoms suggests that ocular lesions are subjectively not more disfiguring than other head and face lesions.
Our results indicate that patients with POLA-BCC experience significant functional, symptomatic, and emotional distress as a consequence of their disease and these are improved with vismodegib treatment. While the main focus of treatment is to control and perhaps cure POLA-BCC tumors, HRQoL should also be taken into account when choosing among potential treatment modalities. Funding for vismodegib was recently discontinued in several countries because the drug was not deemed cost-effective [21, 22]. Given the present findings, decision-makers might do well to consider excluding patients with POLA-BCC from this directive.
This study has some limitations. First, vismodegib has several very prevalent adverse effects including muscle spasms, alopecia, diarrhea, nausea, and fatigue [23], and we were unable to evaluate their influence on the Skindex-16 domain scores. Second, the Skindex-16 was not developed to evaluate specific ophthalmic manifestations such as decreased vision, dry eyes, and epiphora. We believe that a HRQoL instrument geared solely to patients with POLA-BCC is needed to better represent the functional and emotional toll of the disorder.
This is the first study to examine the HRQoL of patients with POLA-BCC. The main strengths of the study are the largest population of vismodegib-treated patients with POLA-BCC reported in the literature and the extensive follow-up period.
Summary
What was known before
-
Vismodegib (Erivedge®) is emerging as the treatment of choice for locally advanced BCC.
-
Severe disfigurement, blindness, and low vision, which may be a consequence of POLA-BCC, can lead to depression and anxiety.
What this study adds
-
Patients with POLA-BCC experience significant functional, symptomatic, and emotional distress, improving with Vismodegib treatment.
-
Decision-makers might do well to consider excluding patients with POLA-BCC from recent discontinuation of funding for Vismodegib in several countries.
References
Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–9. https://doi.org/10.1056/NEJMra044151.
Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998-2012. JAMA Dermatol. 2015;151:976–81. https://doi.org/10.1001/jamadermatol.2015.1188.
Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–54. https://doi.org/10.1038/nrc2503.
Sekulic A, Mangold AR, Northfelt DW, LoRusso PM. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218–23. https://doi.org/10.1097/CCO.0b013e32835ff438.
Basset-Seguin N, Hauschild A, Kunstfeld R, Grob J, Dreno B, Mortier L, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334–48. https://doi.org/10.1016/j.ejca.2017.08.022.
Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surgical Oncol Clin North Am. 2018;27:675–84. https://doi.org/10.1016/j.soc.2018.05.008.
Forsyth RJ. We have to talk about health-related quality of life. Arch Dis Child. 2018;103:913–4. https://doi.org/10.1136/archdischild-2018-314951.
Blackford S, Roberts D, Salek MS, Finlay A. Basal cell carcinomas cause little handicap. Qual Life Res. 1996;5:191–4. https://doi.org/10.1007/bf00434740.
Hansson J, Bartley K, Karagiannis T, Grob JJ, Kunstfeld R, Dreno B, et al. Assessment of quality of life using Skindex-16 in patients with advanced basal cell carcinoma treated with vismodegib in the STEVIE study. Eur J Dermatol. 2018;28:775–83. https://doi.org/10.1684/ejd.2018.3448.
Yin VT, Pfeiffer ML, Esmaeli B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthalmic Plast Reconstructive Surg. 2013;29:87–92. https://doi.org/10.1097/IOP.0b013e3182831bf3.
Nix NM, Burdine O, Walker M. Vismodegib: first-in-class hedgehog pathway inhibitor for metastatic or locally advanced basal cell carcinoma. J Adv Practitioner Oncol. 2014;5:294–6. https://doi.org/10.6004/jadpro.2014.5.4.7.
Basset-Seguin N, Hauschild A, Grob JJ, Kunstfeld R, Dreno B, Mortier L, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729–36. https://doi.org/10.1016/S1470-2045(15)70198-1.
Ben Ishai M, Tiosano A, Fenig E, Ben Simon G, Yassur I. Outcomes of Vismodegib for periocular locally advanced basal cell carcinoma from an open-label trial. JAMA Ophthalmol. 2020. https://doi.org/10.1001/jamaophthalmol.2020.1539.
Clarke A, Rumsey N, Collin JR, Wyn-Williams M. Psychosocial distress associated with disfiguring eye conditions. Eye. 2003;17:35–40. https://doi.org/10.1038/sj.eye.6700234.
Hamedani AG, VanderBeek BL, Willis AW. Blindness and visual impairment in the medicare population: disparities and association with hip fracture and neuropsychiatric outcomes. Ophthalmic Epidemiol. 2019;26:279–85. https://doi.org/10.1080/09286586.2019.1611879.
Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatologic Clin. 2012;30:231–6. https://doi.org/10.1016/j.det.2011.11.003.
Shingler SL, Garside J, Samanta K, Lear JT, Keohane S, Lloyd AJ. Utilities for advanced basal cell carcinoma. J Med Econ. 2013;16:777–83. https://doi.org/10.3111/13696998.2013.800822.
Steenrod AW, Smyth EN, Bush EN, Chang AL, Arron ST, Helfrich YR, et al. A qualitative comparison of symptoms and impact of varying stages of basal cell carcinoma. Dermatol Ther. 2015;5:183–99. https://doi.org/10.1007/s13555-015-0081-6.
Alon Kahana M. Vismodegib for basal cell carcinoma. In: Kanaga Rajan P, editor. AAO Daily 2019. 2019.
Durnian JM, Noonan CP, Marsh IB. The psychosocial effects of adult strabismus: a review. Br J Ophthalmol. 2011;95:450–3. https://doi.org/10.1136/bjo.2010.188425.
McKee S. Roche’s Erivedge no longer funded by the NHSPharmaTimes online. 2017.
Review P-COD. Final Reccomendation for Vismodegib (Erivedge) for Basal Cell Carcinoma pERC Meeting. January, 2013; Reconsideration Meeting: December 19, 2013.
Frampton JE, Basset-Seguin N. Vismodegib: a review in advanced basal cell carcinoma. Drugs. 2018;78:1145–56. https://doi.org/10.1007/s40265-018-0948-9.
Acknowledgements
We thank all the patients and their families, investigators, and research teams who participated in this study. F. Hoffman-La Roche Ltd, for providing the data from the STEVIE study for analysis.
Author information
Authors and Affiliations
Contributions
AG and AT acquired the data and drafted the manuscript. MB-I and EB analyzed the data and aided in interpreting the results. GBS and IY designed the current study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was a secondary analysis of the STEVIE data and did not involve the recruitment of new participants. Therefore, institutional review board approval and patient consent were waived by our institutional protocol. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gershoni, A., Tiosano, A., Ben Ishai, M. et al. Vismodegib improves quality of life in patients with periocular locally advanced basal cell carcinoma: subgroup analysis, STEVIE trial. Eye 36, 407–413 (2022). https://doi.org/10.1038/s41433-021-01493-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01493-2