Abstract
Purpose
To evaluate the utility of dissolvable collagen punctal plugs (CPP) in reducing ocular surface irritation after intravitreal injections (IVI).
Methods
Sixty-four subjects in the experimental group received CPP after intravitreal injections. Sixty-two controls did not receive CPP. Reductions in the Ocular Surface Disease Index© (OSDI) and Standardized Patient Evaluation of Eye Dryness II (SPEED II) scores were analysed.
Results
Dry eye symptoms, as measured by reductions from the pre- to post-injection OSDI (p = 0.137) and SPEED II (p = 0.381) scores, did not significantly differ between the two groups. In sub-group analysis, patients with objective findings of dry eyes had significant improvement in their symptoms (p = 0.046) with CPP. The effect of CPP is not significant in those without dry eyes (p = 0.27).
Conclusion
CPPs were not effective in reducing post-injection ocular irritation in patients with no or only mild dry eye symptoms. CPPs improved patients’ post-injection comfort levels in those who had moderate-to-severe symptoms and objective findings of dry eye. Though costly CPP could be considered in selective patients. A standardized eye rinse could be a simple, efficacious, and cost-effective way to reduce post-injection ocular irritation; however, more studies are needed.
Similar content being viewed by others
Introduction
Intravitreal injection (IVI) is the most commonly performed ophthalmic procedure (2.5 million in 2011) in the United States [1]. Endophthalmitis secondary to IVI is a rare but serious complication with a rate ranging from 0.028 (~1 out of every 3544 IVIs) to 0.056% (~1 out of every 1779 IVIs) [1, 2].
Povidone-iodine (PVI) has been widely studied as an antiseptic agent and its application is considered the standard of care when preparing for IVI [3,4,5]. PVI is also known to be toxic to the corneal epithelium and delays ocular surface healing [6,7,8]. Although guidelines on pre- and peri-injection antiseptic techniques are well-studied, there has been a relative lack of consensus on ocular surface management to reduce the severity and duration of post-injection pain and discomfort [9,10,11,12]. Patients frequently report post-injection ocular discomfort (e.g., tearing, burning, redness and foreign body sensation) similar to symptoms of dry eye disease [7, 12]. An additional consideration is that due to the demographics of patients receiving IVI, many patients may already have multiple predisposing risk factors for and various degrees of ocular surface disease (OSD). Despite the usage of artificial tears, many IVI patients often report significant post-injection ocular irritation within the first 24 h [7, 12].
Punctal plugs are a well-tolerated and well-described adjunctive therapy in the management of OSD and have been shown to improve dry eye symptoms refractory to topical lubrication alone [13, 14]. The primary objective of the present study is to investigate the utility of dissolvable collagen punctal plugs (CPP) in reducing ocular discomfort and dry eye symptoms after IVI measured by the Ocular Surface Disease Index© (OSDI) and Standardized Patient Evaluation of Eye Dryness II (SPEED II) questionnaires [15]. The secondary outcome is to describe other risk or protective factors associated with ocular surface discomfort after IVI. We are unaware of any reports in the literature that discuss the potential benefits of using punctal plugs for the purpose of decreasing post-injection PVI-related ocular surface irritation symptoms.
Methods
Study design and participants
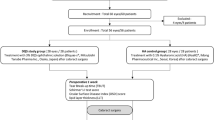
We conducted a single-centre, prospective, randomized-controlled, single-masked clinical trial at the Dean McGee Eye Institute, Department of Ophthalmology, University of Oklahoma Health and Sciences Center, Oklahoma City, OK. This study adhered to the tenets of the Declaration of Helsinki. Ethics approval for this study was obtained from the Institutional Review Board of the University of Oklahoma Health and Sciences Center (IRB #9810) and the trial is registered with the Clinical Trials registry (identifier, NCT03945071). Written informed consent was obtained from all study participants.
Consecutive patients who required IVI for their clinical conditions from two retina specialists at the Dean McGee Eye Institute were identified as potential study participants from September 2019 through March 2020. Exclusion criteria included no prior IVI, current punctal plug use, history of punctal cautery, active or history of any ocular infection, eyelid trauma, eyelid surgery, graft versus host disease or thyroid eye disease. Patients were also excluded if pregnant or were unable or unwilling to participate in the study including answer the post-injection telephone questionnaire 24–72 h after IVI.
Participants were masked and randomly assigned to either the experimental or the control group with an allocation ratio of 1:1. The random allocation sequence was generated by a computer-based random number-producing algorithm that was completed prior to the enrolment of the study.
Procedures
Participants in both the experimental and control groups were asked to complete the OSDI (Appendix 1) and SPEED II (Appendix 2) questionnaires on the REDCap secure web platform on an Apple iPad® (Cupertino, CA) tablet provided by our Clinical Trial Department. Assistance in filling out the questionnaire on the tablet was available when patients either displayed difficulty using the tablet or when they requested assistance to help record their answers on the tablet. Objective testing of the ocular surface consisted of tear break-up time (TBUT), Schirmer test and Oxford fluorescein corneal staining grade [16].
Corneal fluorescein staining grade
A fluorescein strip was wetted with one drop of normal saline before gently dabbed onto the lower palpebral conjunctiva of the studied eye. The patient was asked to blink three times, then hold the eyes open as the staining pattern was examined at the slit lamp using the cobalt blue filter. The corneal fluorescein staining pattern was compared to the Oxford scheme of grading of corneal and conjunctival staining by Bron et al. and the grading was documented [17]. Dry eye disease was defined as fluorescein staining grade >1.
Tear break-up time (TBUT)
Immediately after the corneal fluorescein staining grade was documented, patients were asked to blink three times then hold the eyes open while the TBUT was documented in seconds. Dry eye disease was defined as TBUT < 10 s.
Schirmer test
A strip of filter paper was placed on the inner aspect of the lateral palpebral fissure of the lower fornix of the studied eye for 5 min. Patients were told to gently close their eyes during this part of the exam. The amount of wetting on the filter paper (mm) at the end of the 5 min was documented. Dry eye disease was defined as a wetting distance of <5 mm.
After the completion of objective assessment of dry eye (corneal fluorescein staining, TBUT and Schirmer test) and subjective questionnaires (OSDI and SPEED II), patients underwent standardized preparation for IVIs. One drop of proparacaine was instilled in the studied eye followed by one drop of 5% PVI, and this process was repeated two more times for a total of three rounds of proparacaine followed by 5% PVI. All patients received IVIs within 15 min of the last PVI instillation. A closed-lip lid speculum was used to hold the eyelids open for the IVIs. After the injection was performed, 20 mL of balanced salt solution were used to thoroughly rinse the ocular surface. In the experimental group, the patient’s lower punctum of the studied eye was first dilated with a punctal dilator, followed by the placement of a dissolvable CPP (Oasis®, Glendora, CA USA) sized in either 0.3- or 0.4-mm diameter × 2-mm length using Jeweller forceps. Patients in the control group received the same punctal dilation, but an empty Jeweller forceps touched the lower punctal opening of the studied eye and no CPP was inserted. Post-injection OSDI and SPEED II questionnaires were administered between 24 and 72 h later over the phone.
OSDI and SPEED II questionnaires
The OSDI is assessed on a scale of 0–100, with a higher score representing greater disability. We used OSDI to define ocular surface as having no dry eye disease (0–12 points), mild (13–22 points) dry eye disease, moderate (23–32 points) dry eye disease or severe (33–100 points) dry eye disease [18]. We also used SPEED II as an additional measurement of dry eye symptoms. SPEED II is assessed on a scale of 0–28, with a higher score representing greater disability. A SPEED II score >19 suggests symptomatic dry eye disease [15]. No published range is currently available for SPEED II questionnaires to stratify dry eye disease severity level. OSDI and SPEED II have similar reliability coefficient and may be used as a measure of dry eye severity in clinical practice and epidemiological studies [18, 19].
Power analysis and statistical evaluation
Sample size estimation was performed based on the outcome of reduction in OSDI scores (pre-injection scores minus post-injection scores). Based on our preliminary data from 25 patients (13 control, 12 experimental), mean (SD) reduction in OSDI score in the control group and experimental group was 7.1 (13.9) and 19.7 (23.1), respectively. To detect a between-groups difference of 12 (deemed clinically important) in the outcome with 90% power, we estimated to enrol 55 patients per group based on a two-sided two-sample t-test with unequal variances and 0.05 alpha level.
Descriptive statistics were used to summarize basic demographic characteristics between the intervention and control groups. Continuous data were summarized using mean (SD) by group. Categorical data were summarized using count (percent) by group. Comparisons between treatment groups were performed using the Chi-square test for categorical variables and two-sample t-test for continuous variables. Multivariate analyses were conducted using linear regression, where the following baseline variables were considered: age, gender, race, diagnosis, diabetes type, insulin dependence, length of diabetes, HgA1c level, using eye drops for lubrication, fluctuating vision, blepharitis, fluorescein staining, TBUT, Schirmer’s test, OSDI and SPEED II scores. Two-way interactions between each of these baseline variables and treatment group were assessed. Non-significant terms were removed based on the backward variable selection method. Analyses were performed in SAS version 9.4 (SAS Institute, Inc., Cary, NC). All p values were considered statistically significant when they were <0.05.
Results
A total of 126 patients (64 in the experimental group and 62 in the control group) participated in the study and 90.5% (59 in experimental group and 55 in the control group) successfully completed the study. Overall, the average age was 70.8 (SD = 13.2; range = 33–93) years and 52.4% were female. The study population was an accurate reflection of demographic distributions of patients that presented to our institution for IVIs. Baseline demographic information of both groups showed no statistically significant difference (Table 1). No post-injection endophthalmitis or any other complications occurred in participants during the study.
There were no significant differences in the baseline objective and subjective evaluations of dry eye state between the two groups (Table 2). Overall, there was no significant difference in the mean OSDI score reduction between the punctal plug group (15.3, SD = 17.7) and the control group (10.7, SD = 14.3) (p = 0.137, Table 2). Similarly, there was no significant difference in the mean SPEED II score reduction between the punctal plug group (1.8, SD = 6) and the control group (0.9, SD = 5.3) (p = 0.381, Table 2).
Final multivariate analysis revealed no difference between the experimental and control groups in the outcome of OSDI score reduction (p = 0.42), after adjusting for pre-injection OSDI score. However, patients in the moderate/severe dry eye category based on pre-injection OSDI scores had a significantly higher reduction (p < 0.0001) in post-injection OSDI scores compared to those in the normal/mild dry eye category, regardless of receiving CPP or not (Table 3).
Final multivariate analysis of the outcome of SPEED II score reduction revealed that the effect of CPP differed depending on patient’s baseline dry eye status defined by objective fluorescein staining (p = 0.03). Dry eye patients (Oxford fluorescein grade >1) who received CPP had a significantly higher reduction (p = 0.046) in the SPEED II scores compared to those who did not receive a CPP, while the effect of CPP is not significant in those without dry eyes (p = 0.27). After controlling the treatment effect, dry eye patients who answered ‘frequently’ or ‘a lot/always’ to the question ‘do you have fluctuating vision that can be corrected with blinking?’ had a significantly higher reduction (p < 0.0001) in the post-injection SPEED II scores than those who answered ‘never’ or ‘sometimes’, but this difference was not significant among patients without dry eyes at baseline (Table 4).
There were no significant associations (all p > 0.05) between TBUT, Schirmer test, use of artificial tears and either outcome (reduction in OSDI and SPEED II scores).
Discussion
IVI can be associated with significant level of ocular pain and discomfort [7, 8, 20]. Up to a quarter of IVI patients experience a high level of anxiety (Visual Analogue Scale for Anxiety >6) and up to 10% experience severe pain (Visual Analogue Scale for Pain >6), which can lead to treatment discontinuation and potential vision loss [20, 21]. The reported post-injection ocular symptoms often closely resemble those of dry eye disease [8, 12]. Therefore, retinal specialists may be increasingly motivated to improve the IVI experience for patients to both improve treatment adherence and enhance patients’ subjective experiences.
PVI is comprised of diatomic iodine and polyvinylpyrrolidone (povidone) and exhibits its antiseptic effect via oxidation and disruption of bacterial and viral membrane, cytologic proteins and fatty acids [1, 3]. Numerous studies have concluded that IVIs may cause mild pain regardless of the topical anaesthetic agent used [22, 23]. While peri-injection pain management is important, we suggest that ocular surface irritation in the immediate post-injection period can contribute significantly to patients’ overall comfort and IVI experience. Several studies have investigated PVI’s toxicity to the ocular surface [24, 25]. In addition to PVI usage, inadequate rinsing of the ocular surface may further contribute to post-injection discomfort.
Dissolvable CPPs are often used clinically as a trial prior to insertion of permanent silicone punctal plugs for dry eye patients. Punctal plugs alleviate dry eye symptoms by increasing the tear lake and therefore improve both aqueous tear deficiency and accelerated aqueous evaporation. In addition, human tears contain growth factors and cytokines that lubricate, heal and protect the ocular surface from infections and irritants [26, 27]. A healthy quantitative and qualitative tear film has the potential to not only dilute residual PVI, but also promote corneal epithelial healing post-injection [28].
To the best of our knowledge, this study is the first to evaluate the utility of using dissolvable CPPs to reduce post-IVI irritation. We found that the use of dissolvable CPPs in all IVI patients is not effective in reducing post-injection ocular irritation; however, they are effective in reducing post-injection ocular irritation (measured by SPEED II scores) in those with objective findings of dry eyes, defined by Oxford staining grade >1. The effectiveness of CPPs was most pronounced in patients with moderate–severe dry eye disease (as per the baseline OSDI scores). The use of CPPs in patients without dry eyes (Oxford staining grade <1) is not indicated as it actually increased the post-injection SPEED II score, suggesting that this subgroup of patients had experienced more ocular surface irritation with CPPs. One explanation for this trend is that the increase in tear lake and presence of CPP could have decreased ocular comfort and vision quality in those without dry eyes. In addition, the cost of CPPs is ~$250 per application ($100 for CPPs and $151 professional service fee). Although longer-lasting punctal plugs could be placed in patients with severe dry eye disease, the total cost of punctal plugs to the health care system will need to be carefully evaluated in future studies.
Furthermore, after controlling for treatment, patients who showed moderate and severe dry eyes based on pre-injection OSDI also showed significant reductions in their post-injection OSDI scores, regardless of receiving a CPP or not. Similarly, regardless of CPP placement, patients who reported ‘frequently’ and ‘a lot/always’ having fluctuating vision that corrects with blinking showed significant reductions in the SPEED II scores. We postulate the reductions in OSDI and SPEED II scores could be secondary to the placebo effect and/or our generous 20-mL rinsing protocol, which may greatly reduce residual PVI on ocular/peri-ocular surface.
While most guidelines on IVI have focused on how to reduce peri-injection pain and minimize post-injection endophthalmitis [22, 23, 29], there is no evidence-based consensus on post-injection eye rinse techniques [4, 11, 29, 30]. Jandorf et al. concluded that 3-mL irrigation caused significant less corneal epithelial staining, but it did not reduce patient discomfort [30]. The practice pattern of post-injection eye rinse also differs among the retinal specialists, even at our eye institute. There is no standardized post-IVI eye rinse protocol, and some retina specialists do not perform post-IVI eye wash. This points to the need for future studies comparing the effectiveness, quantity and techniques of post-injection eye rinse to post-injection comfort level.
Strengths of our study include the prospective, randomized-control and single-masked study design, a baseline objective assessment of the ocular surface, and the pre- and post-injection questionnaires. The post-injection questionnaires were done within 72 h to minimize recall bias. We also achieve relatively high retention rate of the study participants to minimize attrition bias. Of the 126 enroled participants, 114 (59 in the experimental group and 55 in the control group) followed through to the end of the study (90.5% retention rate) allowing us to achieve our pre-study power calculations.
Limitations to the study include selection bias; since enrolment was voluntary, patients with no or mild dry eye symptoms may have declined to enrol. In addition, only subjective questionnaires were administered to assess post-injection ocular surface status rather than repeat objective assessment. Future studies may wish to incorporate objective ocular surface assessment after the IVIs and/or include other objective parameters such as tear osmolality, inflammatory markers and the extent of blepharitis and meibomian gland function. Finally, investigating the effect of different quantities of normal saline eye rinse on patients’ comfort level may also be considered.
In conclusion, the universal use of CPPs on all patients presenting for IVI was not effective in reducing post-injection ocular irritation. In sub-group analysis, CPPs did improve patients’ post-injection comfort level in those who had moderate-to-severe dry eye symptoms and objective dry eye findings on corneal fluorescein staining. But the high cost burden of CPPs on health care system needs to be carefully evaluated against its benefits. CPPs are not indicated in those who do not or only exhibit mild symptoms of dry eye and could even potentially cause increased discomfort in these patients. Last, copious rinsing of the ocular surface could be a more economical practice to independently improve patients’ post-injection comfort level, but this requires further study for confirmation. Retina specialists who perform IVIs may wish to consider the results of this study as it relates to their respective clinical practices.
Summary
What was known before
-
Povidine-Iodine can cause ocular surface irritation after IVIs.
What this study adds
-
Certain patients benefit from dissolvable CPP when receiving IVIs prepared by the PVI solutions.
References
Merani R, Hunyor AP. Endophthalmitis following intravitreal anti-vascular endothelial growth factor (VEGF) injection: a comprehensive review. Int J Retina Vitreous. 2015;1:9.
Patel SN, Gangaputra S, Sternberg P, Jr, Kim SJ. Prophylaxis measures for postinjection endophthalmitis. Surv Ophthalmol. 2020;65:408–20.
Grzybowski A, Kanclerz P, Myers WG. The use of povidone-iodine in ophthalmology. Curr Opin Ophthalmol. 2018;29:19–32.
Avery RL, Bakri SJ, Blumenkranz MS, Brucker AJ, Cunningham ET, D’Amico DJ, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1–18.
Hinkle JW, Wykoff CC, Lim JI, Hahn P, Kim SJ, Tabandeh H, et al. “Iodine allergy” and the use of povidone iodine for endophthalmitis prophylaxis. J Vitreoretina Dis. 2020;4:65–8.
Wykoff CC, Flynn HW Jr, Han DP. Allergy to povidone-iodine and cephalosporins: the clinical dilemma in ophthalmic use. Am J Ophthalmol. 2011;151:4–6.
Laude A, Lim JW, Srinagesh V, Tong L. The effect of intravitreal injections on dry eye, and proposed management strategies. Clin Ophthalmol. 2017;11:1491–7.
Saedon H, Nosek J, Phillips J, Narendran N, Yang YC. Ocular surface effects of repeated application of povidone iodine in patients receiving frequent intravitreal injections. Cutan Ocul Toxicol. 2017;36:343–6.
Chaturvedi R, Wannamaker KW, Riviere PJ, Khanani AM, Wykoff CC, Chao DL. Real-world trends in intravitreal injection practices among american retina specialists. Ophthalmol Retina. 2019;3:656–62.
Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the united states. Am J Ophthalmol. 2011;151:329–32.
Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E, Loewenstein A, et al. 2018 update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmologica. 2018;239:181–93.
Ridder WH 3rd, Oquindo C, Dhamdhere K, Burke J. Effect of povidone iodine 5% on the cornea, vision, and subjective comfort. Optom Vis Sci. 2017;94:732–41.
Marcet MM, Shtein RM, Bradley EA, Deng SX, Meyer DR, Bilyk JR, et al. Safety and efficacy of lacrimal drainage system plugs for dry eye syndrome: a report by the american academy of ophthalmology. Ophthalmology. 2015;122:1681–7.
Jehangir N, Bever G, Mahmood SM, Moshirfar M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J Ophthalmol. 2016;2016:9312340.
Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32:1204–10.
Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19:644–9.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50.
Miller KL, Walt JG, Mink DR, Satram-Hong S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128:94–101.
Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular surface disease index (OSDI) versus the standard patient evaluation of eye dryness (SPEED): a study of a nonclinical sample. Cornea. 2016;35:175–80.
Segal O, Segal-Trivitz Y, Nemet AY, Cohen P, Geffen N, Mimouni M. Anxiety levels and perceived pain intensity during intravitreal injections. Acta Ophthalmol. 2016;94:203–4.
Vaze A, Fraser-Bell S, Gillies M. Reasons for discontinuation of intravitreal vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration. Retina. 2014;34:1774–8.
Blaha GR, Tilton EP, Barouch FC, Marx JL. Randomized trial of anesthetic methods for intravitreal injections. Retina. 2011;31:535–9.
Davis MJ, Pollack JS, Shott S. Comparison of topical anesthetics for intravitreal injections: a randomized clinical trial. Retina. 2012;32:701–5.
Dohlman TH, Lertsuwanroj B, D’Amico DJ, Ciralsky JB, Kiss S. Evaluation of signs and symptoms of ocular surface disease after intravitreal injection. Acta Ophthalmol. 2019;97:e1154–e56.
Shibata Y, Tanaka Y, Tomita T, Taogoshi T, Kimura Y, Chikama T, et al. Evaluation of corneal damage caused by iodine preparations using human corneal epithelial cells. Jpn J Ophthalmol. 2014;58:522–7.
Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf. 2007;5:228–39.
Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome: summary of a cochrane systematic review. Br J Ophthalmol. 2019;103:301–6.
Pflugfelder SC. Tear dysfunction and the cornea: LXVIII edward jackson memorial lecture. Am J Ophthalmol. 2011;152:900–9.e1.
Crabb MG, Liu E, Freeman A, Hsu T, Supramaniam D, Kaidonis G, et al. The intravitreal injection pain study: a randomized control study comparing subjective pain with injection technique. Acta Ophthalmol. 2019;97:e1153–4.
Jandorf S, Krogh Nielsen M, Sorensen TL. Irrigating the eye after intravitreal injection reduces epithelial damage but not patient discomfort. Acta Ophthalmol. 2019;97:e670–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, H.D., Surbeck, J.W., Marsh, H.R. et al. The effect of punctal plugs in reducing ocular surface irritation after povidone-iodine preparation of intravitreal injection—a randomized trial. Eye 36, 568–574 (2022). https://doi.org/10.1038/s41433-021-01476-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01476-3
This article is cited by
-
The effect of ocular rinse volume on surface irritation after povidone-iodine preparation for intravitreal injections: a randomized controlled trial
International Journal of Retina and Vitreous (2023)