Abstract
Introduction
Retinopathy of prematurity (ROP) is the most common disease leading to blindness in extreme preterm infants. Current screening guidelines recommend frequent eye examinations. There is a dearth of trained ophthalmologists for these frequent screening procedures. The ANZNN neonatal network report (2013) found that only 6.4% of all screened infants had severe ROP and less than half received treatment. WINROP (online prediction model, Sweden) uses the postnatal weight gain (surrogate marker for low insulin-like growth factor IGF-1 and poor retinal vascular growth) to identify ROP requiring treatment and aims to reduce the number of examinations. Our objective was to validate the WINROP model in an Australian cohort of preterm infants.
Methods
Birth weight, gestational age, and weekly weight measurements were retrieved retrospectively along with the final ROP outcomes and plotted on the online WINROP software.
Results
The sensitivity, specificity, positive predictive value, and negative predictive value of WINROP were 85.7%, 59.0%, 6.98%, and 99.1% respectively for a cohort of 221 preterm infants (Median birth weight, 1040 g; Gestational age, 27.9 weeks). WINROP alarm was signaled in 42.6% of all infants. WINROP did not signal an alarm in one infant who needed treatment. This infant had intra ventricular hemorrhage grade 3–4 and temporary ventricular dilatation.
Conclusions
This is the first Australian study validating WINROP model. Our findings suggest that it lacked sensitivity to be used alone. However, adjusting the algorithm for the Australian population may improve the efficacy and reduce the number of examinations when used along with the current screening guidelines.
Similar content being viewed by others

Introduction
Retinopathy of prematurity (ROP) is the most common disease leading to childhood blindness among preterm infants [1,2,3]. ROP is characterized by pathological retinal neo-vascularization in the maturing retina, detectable weeks after preterm birth. Preterm infants are exposed to repeated ophthalmological examinations to identify ROP requiring treatment, defined as ROP type 1 according to ETROP guidelines [4]. Current ROP screening is based on a simple prediction model with two dichotomized predictors, birth weight (BW) and gestational age at birth (GA). The Australia and New Zealand guideline recommends that all infants with BW under 1250 g or gestation under 31 weeks should be screened for ROP [5]. Current ROP screening guidelines have high sensitivity, but low specificity. In fact, very few preterm infants examined require treatment, based on multiple large studies [6,7,8]. The Australian data suggest that only 6.3% of total screened infants had severe ROP (stage 3 and above) and less than half of these infants with severe ROP received treatment [5, 9]. Further, repeated ophthalmological examinations lead to stress and discomfort in these fragile preterm infants even when performed by an experienced ophthalmologist [10]. These screening sessions are time consuming and uncomfortable. They also cause stress and anxiety to parents. Further, there is a dearth of experienced ophthalmologists in both the high-income and low-income countries to carry out these ROP examinations.
There is some recent evidence to support use of prediction models that include postnatal weight gain which may potentially reduce the number of infants requiring examinations while still accurately identifying infants who require treatment [11,12,13,14,15,16]. The scientific rationale is that low postnatal weight gain acts as a surrogate marker for a slower-than expected rise in serum insulin-like growth factor-1 (IGF-1), resulting in an insufficient activation of retinal vascular endothelial growth factor by IGF-1 and poor retinal vascular growth [17, 18].
Based on the above rationale, an online prediction model, WINROP, was developed in Sweden [19, 20]. By recording the infant’s BW and GA along with weekly weight measurements, this WINROP model accumulates and calculates the infant’s risk of developing severe ROP requiring treatment. It also has an alarm function that signals if an infant is at high risk of developing severe ROP requiring treatment. The infants’ data is recorded anonymously in the online system, identified only by WINROP identification number. WINROP model is developed as a supplement rather than being a substitute to established ROP screening examination. It aims to safely minimize the number of ROP screening examinations in infants at low risk of ROP requiring treatment and to alert physicians to pay special attention to infants who are at high risk. The WINROP model has been validated in several Swedish cohorts with high sensitivity as well as in other high and low-income countries [11,12,13,14,15,16]. However, the sensitivity and specificity of the WINROP model varies in these different cohorts; reflecting the inherent characteristics of the preterm infants and the neonatal care rendered to them in these diverse setups. It has been suggested that the WINROP model should be validated in different populations across the world. The aim of the present study was to validate the WINROP prediction model in Australian preterm infants.
Materials and methods
This was a retrospective study carried out in a level 3 NICU of a tertiary hospital in Western Australia to evaluate the ability of an online postnatal weight-gain prediction model (WINROP) to identify severe ROP in an Australian preterm population.
Objective
The objective was to measure the sensitivity, specificity, positive, and negative predictive values of WINROP identifying severe ROP, requiring treatment in an Australian preterm population.
WINROP model
The use of WINROP prediction model requires that the infant’s GA to be from 23 to 32 gestational weeks at birth, weekly weight measurements, and physiological weight gain. Infants with incomplete data regarding BW, GA, or final ROP outcome were excluded. Further, infants with incomplete weekly weight measurements or if the weight measurements were judged to inaccurately reflect physiological postnatal weight gain (e.g., when the weight reflects accumulated fluid, as in hydrocephalus) were also excluded from the study. The preterm infants, who met the above criterion, in King Edward Memorial Hospital, Western Australia for a period of 3 years (January 2014 and December 2016), were entered into WINROP prediction model (https://winrop.com/). The infants’ BW, GA, and weekly weight measurements until the postmenstrual age of 35 weeks were retrieved from the database along with the final ROP outcomes. ROP was classified according to Early treatment of ROP (ETROP trial) into type 1 or type 2 or non-type 1/2 or no ROP, based on the zone involved and the staging as per the revised International Classification of ROP [4, 21]. All treatments were done according to the ETROP study guidelines [4]. Data were anonymously recorded in the WINROP system, by an investigator unaware of the final ROP outcome. The WINROP outcome was an alarm or not and was recorded in a separate data file. An alarm means that the infant is at risk for developing severe ROP (requiring treatment according to ETROP guidelines). In this separate data file, the infants ROP outcome were added and calculations performed. No additional interventions were necessary on the participants. ROP screening was continued till treatment was required or complete vascularization of retina occurred.
Statistical analysis
All analyses were performed in SPSS version 25 (IBM, Armonk, NY, USA). Based on actual ROP outcome, the sensitivity and specificity of WINROP alarm in predicting severe ROP was calculated. The prevalence of severe ROP in the study cohort was further used to calculate the negative and positive predictive values of WINROP. Overall, 95% confidence intervals (CIs) were calculated.
Results
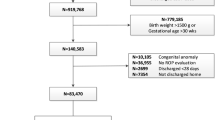
The study included data on 221 preterm infants (123 males and 98 females). The study flow chart has been depicted in Fig. 1. A total of 19 infants had to be excluded due to various reasons as per the study criteria and have been depicted in the study flow chart (Fig. 1). A total of 202 infants were finally included in the analysis. None of these infants had nonphysiological weight gain. The median BW was 1040 g (range, 459–1915 g), and median GA was 27.9 weeks (range, 23.4–31.9 weeks) in the included infants. A detailed BW and GA distribution of these infants with their ROP outcomes is given in Table 1. All included infants completed their final ROP examination. No ROP was detected in 129 infants (63.9%), and less severe ROP was diagnosed in 64 infants (31.7%) and 9 infants (4.45%) received ROP treatment with laser for severe ROP. Of the nine infants receiving treatment, seven infants fulfilled treatment criteria, i.e., developed ROP type 1, and however two infants developing ROP type 2 also received treatment in view of findings on examination suggestive of pre-plus disease. Infants’ median postmenstrual age for first ROP treatment was 36.4 weeks (range, 33.3–44.6 weeks). None of the preterm infants had developed Aggressive Posterior ROP.
ROP alarm was signaled in 86 (42.6%) of all infants and in six of the seven infants developing type 1 ROP (85.6%). In all infants receiving an alarm the median postmenstrual age week for alarm was 30.5 weeks (range 27–35 weeks) and median time to alarm was 2.5 weeks from birth (range, 0–11 weeks). Fifty-nine (68.6 %) of the infants received an alarm within the first 3 weeks of life, Fig. 2. In infants developing ROP type 1 and receiving treatment the median postmenstrual age week for alarm was 27.0 weeks (range 27–32 weeks) and median time to alarm was 3.5 weeks from birth (range, 2–9 weeks). The median time from alarm to treatment for Type 1 ROP was 7.8 weeks (range, 1–11 weeks). The sensitivity and specificity of WINROP for type 1 ROP were 85.7% (42.0–99.2) and 59.0% (51.7–65.9%), respectively. The positive predictive value was 6.98% (2.88–15.1) and negative predictive value (NPV) was 99.1% (94.6–99.9), (Table 2).
By using the WINROP algorithm, there could have been 17.7% reduction in number of direct ophthalmic examinations and around 30% of the total examinations could have been safely delayed in our cohort. This approach did not miss any Type 1 or Type 2 ROP.
WINROP did not signal an alarm in one infant diagnosed and treated for type 1 ROP. This infant developed intra ventricular hemorrhage (IVH) grade 3–4 and temporary ventricular dilatation, which resolved after resolution of clot. WINROP did not signal with an alarm in two infants with ROP type 2; ROP stage 3 in zone II without plus disease. These infants did not have any unphysiological weight gain.
Discussion
Earlier studies have shown that prolonged early IGF-1 deficits and slow postnatal weight gain are associated with a higher risk of severe ROP. Serum IGF-1 levels correlate with fetal and postnatal growth, so postnatal weight gain is a good surrogate marker for serum IGF-1 [17,18,19]. Clinical prediction models such as WINROP, using postnatal weight gain, have been used to identify preterm infants with risk of severe ROP requiring treatment in different setups around the world [11,12,13,14,15,16].
To our knowledge, this is the first study from Australia using a weight gain-based online model for prediction of severe ROP. In the present study, the sensitivity of the WINROP alarm for type 1 ROP was 85.7% which is comparable with the previously mentioned literature.
In this retrospective study, WINROP algorithm did not identify an infant with suspicious nonphysiological weight gain due to temporary ventricular dilatation. This emphasizes the importance of clinical judgment when prospectively recording the infants’ weekly weight measurements in WINROP. WINROP demonstrated a very high sensitivity for detecting severe ROP in some high-income countries: 100% in a Swedish (353 infants) and an American cohort (318 infants) [12, 22]. However, when WINROP model was studied in some developing countries, the sensitivity ranged from a low 55% in a Mexican cohort (352 infants) to being 91% in a Brazilian cohort (366 infants) [13, 23]. In the present study, the specificity was 59%. The highest specificity of 81.7% was noted in the American cohort [12]. Due to low specificity and high false-positive rate, there is a need to do ROP screening as usual for infants with positive alarm. The differences in these values could be partly explained by the varying diversity of each of the cohorts studied in different parts of the world. It also partly reflects the differences in the perinatal and postnatal care in the different parts of the world. Alarm was triggered at birth in six infants. Most alarms (68.6%) occurred in the first 3 weeks after birth and the median time from alarm to treatment was around 8 weeks. This was similar to the study done in India where they had enrolled 70 preterm infants [24].
By using the WINROP algorithm, there could have been 17.7% reduction in number of direct ophthalmic examinations and around 30% of the total examinations could have been safely delayed in our cohort, even without missing a single case of type 2 ROP. This is similar to the studies done previously in the developed world [12, 22].
The present study has a few limitations. First, for any prediction model to be considered robust for screening, the CIs should be narrow. However, the CIs for both sensitivity and specificity in the present study were not narrow enough for it to be considered for routine use. Second, the overall specificity of WINROP alarm was low due to a high false-positive rate. The positive predictive value was very low at 6.98%. Hence, infants with positive alarm would need repeated ROP screening as usual. Third, this was a single centered retrospective study.
Our study has few merits such as having infants encompassing all GAs in a tertiary care setup. The WINROP prediction model in the present study had a very high NPV, thus could potentially be helpful in reducing the number of infants needing repeated ROP screening.
In light of the present study findings, the authors would suggest that the WINROP model could be used alongside the standard ROP screening criteria, rather than replacing it. This could potentially help in changing the examination frequency or timing based on predicted risk. In future, more multicentric studies with larger sample size in different tertiary care neonatal units in Australia are warranted to validate the above findings. Further, the results could be improved by modifying the WINROP model depending upon the different population characteristics.
In this study the WINROP model had a moderately high sensitivity of 85.7% and a very high NPV of 99%. Hence, it could potentially be used along with the current ROP screening criteria to reduce the number of ROP examinations needed in high-risk infants. However, more multicentric studies are needed before modifying existing guidelines.
Summary
What was known before
-
Current ROP screening guidelines (based on birth weight and gestational age) have high sensitivity, but low specificity. This leads to screening a large number of infants with very few needing treatment (Australian data: only 6.3% of total screened infants had severe ROP and <50% of these infants received treatment).
-
Repeated ophthalmological examinations can be stressful to fragile preterm infants and their parents.
What this study adds
-
The WINROP model had a moderately high sensitivity of 85.7% and a very high negative predictive value of 99% in an Australian preterm infant cohort.
-
It could potentially be used along with the current ROP screening criteria to reduce the number of ROP examinations needed in high-risk infants.
References
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82.
Fortes Filho JB, Eckert GU, Procianoy L, Barros CK, Procianoy RS. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye. 2009;23:25–30.
Quinn GE. Retinopathy of prematurity in Brazil: an emerging problem. JPediatr. 2007;83:191–3.
Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Darlow BA. Retinopathy of prematurity: new developments bring concern and hope. J Paediatr Child Health. 2015;51:765–70.
Chiang MF, Arons RR, Flynn JT, Starren JB. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004;111:1317–25.
Lee SK, Normand C, McMillan D, Ohlsson A, Vincer M, Lyons C. Evidence for changing guidelines for routine screening for retinopathy of prematurity. Arch Paediatr Adolesc Med. 2001;155:387–95.
Haines L, Fielder AR, Scrivener R, Wilkinson AR. Retinopathy of prematurity in the UK I: the organisation of services for screening and treatment. Eye. 2002;16:33–8.
Chow SSW. Report of the Australian and New Zealand Neonatal Network 2012. Sydney: ANZNN; 2014. https://npesu.unsw.edu.au/data-collection/australian-new-zealand-neonatalnetwork-anznn#Previous. Reports ANZNN.
Kleberg A, Warren I, Norman E, Morelius E, Berg AC, Mat-Ali E, et al. Lower stress responses after newborn individualized developmental care and assessment program care during eye screening examinations for retinopathy of prematurity: a randomized study. Pediatrics. 2008;121:E1267–78.
Wu C, Lofqvist C, Smith LEH, VanderVeen DK, Hellstrom A, Consortium W. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130:992–9.
Wu C, VanderVeen DK, Hellstrom A, Lofqvist C, Smith LEH. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2010;128:443–7.
Zepeda-Romero LC, Hard AL, Gomez-Ruiz LM, Gutierrez-Padilla JA, Angulo-Castellanos E, Barrera-de-Leon J, et al. Prediction of retinopathy of prematurity using the screening algorithm winrop in a Mexican population of preterm infants. Arch Ophthalmol. 2012;130:720–3.
Zepeda-Romero LC, Lundgren P, Gutierrez-Padilla JA, Gomez-Ruiz LM, Quiles Corona M, Orozco-Monroy JV, et al. Oxygen monitoring reduces the risk for retinopathy of prematurity in a mexican population. Neonatology. 2016;110:135–40.
Choi JH, Lofqvist C, Hellstrom A, Heo H. Efficacy of the screening algorithm winrop in a korean population of preterm infants. JAMA Ophthalmol. 2013;131:62–6.
Piyasena C, Dhaliwal C, Russell H, Hellstrom A, Lofqvist C, Stenson BJ, et al. Prediction of severe retinopathy of prematurity using the winrop algorithm in a birth cohort in south east scotland. Arch Dis Child Fetal Neonatal Ed. 2014;99:F29–33.
Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–8.
Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999;5:1390–5.
Lofqvist C, Andersson E, Sigurdsson J, Engstrom E, Hard AL, Niklasson A, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–18.
Loqvist C, Hansen-Pupp I, Andersson E, Holm K, Smith LEH, Ley D, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulin-like growth factor I. Arch Ophthalmol. 2009;127:622–7.
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Hellström A, Hård AL, Engström E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–45.
Hård AL, Löfqvist C, Fortes Filho JB, Procianoy RS, Smith L, Hellström A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch Ophthalmol. 2010;128:1432–6.
Sanghi G, Narang A, Narula S, Dogra MR. WINROP algorithm for prediction of sight threatening retinopathy of prematurity: initial experience in Indian preterm infants. Indian J Ophthalmol. 2018;66:110–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the institutional ethics committee.
Informed consent
Since this was a retrospective database study, the participants were at no risk and the patient identity was kept confidential during the analyses. Hence, the ethics committee waived off the parental informed consent clause.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desai, S., Athikarisamy, S.E., Lundgren, P. et al. Validation of WINROP (online prediction model) to identify severe retinopathy of prematurity (ROP) in an Australian preterm population: a retrospective study. Eye 35, 1334–1339 (2021). https://doi.org/10.1038/s41433-020-1094-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1094-7