Abstract
Background
The aim of this study is to evaluate the optic nerve sheath diameter (ONSD) in eyes with dysthyroid optic neuropathy (DON) and its relationship with clinical characteristics and disease severity.
Methods
Patients diagnosed as thyroid-associated ophthalmopathy (TAO), with or without DON, and healthy participants were recruited. Vertical and horizontal sectional images of the optic nerve were collected by B-scan ultrasonography. ONSDs at 3 mm and 6 mm behind the eyeball were measured independently by two researchers. Multivariate regression analysis was performed to evaluate the association of ONSD with demographic and ocular parameters in TAO patients. Areas under the receiver operating characteristic curves (AUROCs) were applied to evaluate the diagnostic accuracy of ONSD for DON.
Results
A total of 47 healthy eyes, 36 TAO eyes without DON, and 33 eyes with DON were studied. ONSDs at 3 mm and 6 mm of DON eyes were significantly higher than those in non-DON and healthy eyes (all P < 0.05). There was no significant difference in ONSDs between clinically active and inactive eyes (both P > 0.05). DON occurrence showed a positive association with both ONSDs at 3 mm (β = 0.49, 95% CI: 0.14–0.83, P = 0.007) and 6 mm (β = 0.58, 95% CI: 0.20–0.96, P = 0.003). ONSDs at 3 mm and 6 mm showed a desirable diagnostic capacity to distinguish DON from non-DON eyes (AUROC was 0.77 and 0.75, respectively).
Conclusions
An increase in ONSD is evident in DON eyes independent of clinical activity. Ultrasound-based ONSD has sufficient ability to distinguish DON from non-DON eyes.
Similar content being viewed by others
Introduction
Thyroid-associated ophthalmopathy (TAO), also called Graves’ ophthalmopathy, refers to a potentially sight-threatening disease characterized by volume expansion of the extraocular muscles (EOMs) and/or adipose tissue. Severe forms of the disease, including corneal ulceration or dysthyroid optic neuropathy (DON), affect ∼3–5% of TAO patients [1]. The visual function of DON patients can be severely impaired in a short period, causing a dramatic decrease in their quality of life. The pathogenesis of DON remains unclear and several hypotheses have been put forward. Direct compression of the optic nerve by enlarged EOMs at the orbital apex is currently the most widely accepted mechanism [2]. In a study conducted by the European Group on Graves’ Orbitopathy (EUGOGO), radiological evidence of apical optic nerve compression was demonstrated in 49 out of 56 eyes with DON [3]. Other causes include optic nerve stretch secondary to proptosis, elevated retrobulbar pressure, inflammation, and changes in orbital blood flow [4].
Anatomically, the optic nerve is separated from the optic nerve sheath (ONS) by a subarachnoid space filled with cerebrospinal fluid. The ONS diameter (ONSD) has been used as a non-invasive surrogate of intracranial pressure (ICP). Due to the high availability and safety of ultrasound equipment, the ultrasonographic measurement of ONSD has gained particular interest in emergency departments for monitoring ICP [5]. Recently, ONSD has also been used as a quantitative indicator of translaminar pressure gradient for exploring the pathogenesis of glaucoma [6] and as an auxiliary parameter facilitating the diagnosis of acute optic neuritis [7, 8]. As DON displays similar structural and functional changes to other optic neuropathies, including defects in visual acuity, visual field (VF) and color vision, and optic disc edema [4], we speculate that ONSD might also be different as well. Such changes may be clinically significant and relate to visual function anomalies in TAO patients.
To the best of our knowledge, ultrasonographic based ONSD changes have not yet been documented in TAO patients, especially in those with DON. In this study, we aimed to determine the ONSD changes in TAO patients and investigate its relationship with the clinical characteristics and severity of the disease. We attempted to find an imaging marker of the optic nerve to assist in the early diagnosis of DON.
Materials and methods
Study population
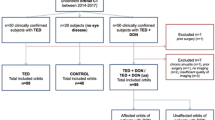
The study protocol was reviewed and approved by the Institutional Review Board of Zhongshan Ophthalmic Center (ZOC). Written informed consent was obtained from all participants. This was a prospective case–control study conducted between April 2019 and July 2019. This study was in agreement with the tenets of the Declaration of Helsinki. Sex-matched participants aged ≥ 18 years were enrolled from the ocular oncology and orbital diseases department of ZOC. Three groups were recruited: TAO patients with DON (DON group), TAO patients without DON (non-DON group), and healthy control subjects (HC group).
The diagnosis of TAO was made according to the Bartley diagnostic criteria for Graves’ ophthalmopathy [9]. The activity and severity of TAO were evaluated in line with the EUGOGO Guidelines [10]. TAO activity was measured by the clinical activity score (CAS). This score is based on whether the following seven items are present: spontaneous retrobulbar pain, pain on attempted upward or downward gaze, redness of eyelids, redness of conjunctiva, swelling of caruncle or plica, swelling of eyelids, and swelling of conjunctiva. A CAS ≥ 3/7 indicates active TAO. The diagnosis of DON was made based on a combination of clinical and radiological findings: decreased best-corrected visual acuity (BCVA) ≥ 2 lines in logMAR visual chart, relative afferent pupillary defect (RAPD) when unilaterally affected, impairment of color vision, VF defect (mean deviation in Humphrey perimetry < −10 dB), abnormal visual-evoked potentials (VEPs) test (latency delay and amplitude reduction), and apical orbital crowding in computed tomography (CT) or magnetic resonance imaging (MRI). Exclusion criteria for the participants were as follows: any diseases affecting ICP, any history of optic neuropathy and glaucoma, severe strabismus impeding ultrasonic optic nerve imaging, and pregnant or breastfeeding women.
Ophthalmic and systemic examination
Detailed medical history, including smoking and glucocorticoid therapy history, was collected before the examination. All subjects underwent a complete ophthalmic and systemic examination including BCVA, applanation tonometry, slit-lamp biomicroscopy, RAPD test, fundoscopy, eye movement evaluation, height, and weight. Pulse and blood pressure were measured after at least 5 min rest. Mean arterial blood pressure (MABP) was calculated as 1/3 × systolic blood pressure + 2/3 × diastolic blood pressure. For all TAO patients, the following ancillary tests were performed: color vision, Hertel exophthalmometry, VF (Humphrey Field Analyzer II 750; Carl Zeiss Meditec, Inc., Dublin, CA, USA), VEP (ESPION; Diagnosys LLC, Inc., Cambridge, UK), orbital CT (Mx8000 IDT; Philips, Amsterdam, The Netherlands), and/or MRI (Achieva 1.5 T; Philips).
Measurement of the optic nerve sheath diameter
All subjects underwent transbulbar B-scan ultrasonography performed by a single investigator (XJ) using a 10 MHz probe (Aviso, Quantel Medical, France) according to a previously published method [11]. In brief, participants lay on the examination bed in a supine position. A sterile, clear sticker was used to cover their eyelids and to keep the study eye closed. The fellow eye was instructed to gaze straight at the mark on the ceiling. Images were captured when the ONS was maximally visualized. Parameters were set the same for all subjects: gain = 100 dB, dynamic range = 60 dB, time gain compensation = 20 dB. A total of four images (two vertical and two horizontal) were collected for each eye. Image J analysis software (available at https://imagej.nih.gov/ij/) was used to measure the ONSDs at 3 mm and 6 mm behind the eyeball. The mean value of the four measurements was summarized as the final ONSD of the study eye. All images were independently analyzed by two researchers (XJ and WX) who were blind to the clinical information of patients. Inter-observer reliability was evaluated with the intraclass correlation coefficient (ICC) and Bland–Altman analysis.
Statistical analysis
Continuous variables were presented as mean ±SD. One-way analysis of variance (ANOVA) was used to analyze the overall difference of the continuous variables among groups. Bonferroni correction was used for multiple comparisons. Univariate and multivariate regression were conducted to analyze the association of ONSD with demographic and ocular parameters. Statistical analysis was performed using GraphPad Prism software (version 7.0.0, GraphPad Software, San Diego, CA). A P-value < 0.05 was considered to be statistically significant. The variances were not significantly different among the groups. Power calculation was not calculated as the study was exploratory.
Results
Demographics
A total of 77 participants were enrolled in this study, with 35 (45.5%) HC group, 19 (24.7%) TAO patients without DON (non-DON group), and 23 (29.9%) TAO patients with DON (DON group). There was no difference in gender distribution (P = 0.596), height (P = 0.796), weight (P = 0.227), and body mass index (P = 0.053) among the three groups (Supplementary Table S1). However, compared with the non-DON and HC group, patients with DON had a higher mean age (P = 0.001) and MABP (P < 0.001). There was a larger proportion of current or ex-smoker in DON group compared with non-DON and HC group (P < 0.05). Eighteen DON patients (78%) and 15 non-DON patients (79%) had glucocorticoid therapy history compared with six HC subjects (17%) (P < 0.001). Eleven DON patients and nine non-DON patients were treated with high-dose intravenous glucocorticoids. Six HC subjects received oral glucocorticoids for the treatment of orbital masses of unenrolled eyes.
Ocular characteristics
There were 47 healthy (37.3%), 36 non-DON (36.5%), and 33 DON (26.2%) eyes included in the analysis. There was no significant difference in spherical equivalent (P = 0.275) among the three groups. Compared with the other two groups, the DON group had significantly worse BCVA and higher intraocular pressure (both P < 0.001). No significant difference was found in proptosis (P = 0.603) between the DON and non-DON groups. Compared with the non-DON group, a higher mean CAS (P < 0.001) was found in the DON group (0.75 ± 0.87 vs. 2.27 ± 1.83; Table 1).
Optic nerve sheath diameter
ICC values showed an excellent agreement between the two researchers (both 0.95 at 3 mm and 6 mm, 95% confidence interval (CI): 0.90–0.98). The Bland–Altman analysis also showed good inter-researcher agreement, with a mean difference of 0.29 mm at 3 mm (95% limit of agreement [LoA]: −0.22 to 0.81) and 0.28 mm at 6 mm (95% LoA: −0.24 to 0.81) (Supplementary Fig. S1).
ONSD measurements of the HC, non-DON, and DON eyes were (5.57 ± 0.54) mm, (5.47 ± 0.59) mm, (6.02 ± 0.53) mm at 3 mm, and (6.57 ± 0.60) mm, (6.39 ± 0.64) mm, (6.92 ± 0.57) mm at 6 mm, respectively (Fig. 1). In one-way ANOVA analysis, statistically significant differences were found both at 3 mm (P < 0.001) and 6 mm (P = 0.002) among the three groups. In the post-hoc pairwise comparison, ONSDs at 3 mm and 6 mm were significantly wider in DON eyes than those in non-DON (both P < 0.05) and HC eyes (both P < 0.05). No significant difference was found between non-DON and HC eyes (P > 0.05).
B-scan ultrasonography in the healthy (a), non-DON (b), and DON eyes (c). The corresponding MRI images of the healthy (d), non-DON (e), and DON eyes (f). Line a represents the axis of the optic nerve. ONSD was measured at 3 mm (line b) and 6 mm (line c) behind the eyeball, perpendicular to line a. ONSD measurements in healthy, non-DON, and DON eyes at 3 mm (g) and 6 mm (h).
To study the effect of disease activity on ONSD, DON eyes were further divided into CAS ≥ 3 group and CAS < 3 group. No significant difference was found in ONSD at either 3 mm or 6 mm (both P > 0.05) between the two groups (Supplementary Table S2).
Correlation of ONSD with demographic and ocular parameters
We analyzed the correlation of ONSD with demographic and ocular parameters in TAO patients (Tables 2 and 3). Univariate regression analysis showed significant positive associations of ONSD with DON occurrence (at 3 mm: β = 0.54, 95% CI: 0.27–0.81, P < 0.001; at 6 mm: β = 0.53, 95% CI: 0.24–0.82, P = 0.001), age (at 3 mm: β = 0.02, 95% CI: 0.01–0.03, P = 0.001; at 6 mm: β = 0.02, 95% CI: 0.01–0.03, P = 0.003), and MABP (at 3 mm: β = 0.02, 95% CI: 0.01–0.03, P = 0.018; at 6 mm: β = 0.02, 95% CI: 0.01–0.04, P = 0.013). Multivariate regression with stepwise estimation demonstrated that DON occurrence (at 3 mm: β = 0.49, 95% CI: 0.14–0.83, P = 0.007; at 6 mm: β = 0.58, 95% CI: 0.20–0.96, P = 0.003) and older age (at 3 mm: β = 0.02, 95% CI: 0.01–0.03, P = 0.033; at 6 mm: β = 0.02, 95% CI: 0.01–0.04, P = 0.011) were significantly associated with larger ONSD.
Diagnostic abilities of the optic nerve sheath diameter
The AUROCs for distinguishing DON eyes from non-DON eyes were displayed in Fig. 2. The AUC was 0.77 and 0.75 at 3 mm and 6 mm, respectively. A cut-off value of 5.792 mm was set for ONSD at 3 mm. Its sensitivity and specificity for diagnosing DON was 66.67% and 75%, respectively.
Discussion
In this study, we found that the ONSD was significantly wider in DON vs. non-DON and healthy eyes. This was independent of clinical activity. Ultrasound-based ONSD measurement showed a desirable diagnostic accuracy in differentiating DON from non-DON eyes. To the best of our knowledge, this is the first study on the ultrasonographic measurement of ONSD in TAO patients.
The optic nerve is enveloped by ONS, with cerebrospinal fluid between. Therefore, ONSD represents the width of the optic nerve plus the subarachnoid space. Several imaging techniques, including ultrasonography, CT, and MRI, have been used to measure ONSD previously [12]. Compared with CT and MRI, ultrasonography has the advantage of being a simple, convenient, and low-cost tool that is also free of radiation. Previous studies have consistently proved a significant correlation between ICP elevation and ONSD widening. Based on this, ultrasonographic measurement of ONSD has been widely used as an indicator of increased ICP in emergency departments [13]. Recently, alterations in ONSD have also been observed in various ophthalmic diseases, especially in optic neuropathies. For example, Jaggi et al. [14] found that ONSD in patients with normal-tension glaucoma was significantly larger than those in HC subjects. Lochner et al. [15] reported that ONSD in acute optic neuritis eyes were significantly thicker than unaffected fellow eyes. In this study, we demonstrated that ONSD in DON eyes was wider, similar to the findings in acute optic neuritis eyes.
The pathogenesis underlying DON remains unclear but might be related to multiple factors, including compression of the optic nerve at the orbital apex, inflammation, elevated retrobulbar pressure, and vascular insufficiency [4, 16]. In theory, elevated retrobulbar pressure would mechanically compress the optic nerve and decrease the diameter of ONS. However, we unexpectedly found that ONSDs at 3 mm and 6 mm significantly increased in DON patients. The results indicated that the proximal end of the optic nerve was not directly compressed by the elevated retrobulbar pressure. Another probable explanation was that the crowded apex in DON patients blocked the reflux of cerebrospinal fluid in the subarachnoid space. In either case, ONSD measurements should be varied along the optic nerve. However, because of the limited scanning depth of B-scan ultrasonography, we cannot confirm this hypothesis in this study. In terms of inflammatory mechanism, our research showed no correlation between CAS and ONSD, suggesting that inflammation did not directly affect ONSD. Therefore, we hypothesized that inflammation might not participate in the development of DON. Another possibility is that CAS might not ideally reflect inflammation of the retrobulbar soft tissue. Besides, age was found to be positively correlated with ONSD in TAO patients.
In this study, it was of note that ONSDs at both 3 mm and 6 mm were significantly wider in DON eyes than in non-DON eyes. We thus assumed that DON might be characterized by ONSD enlargement. In this regard, optic nerve ultrasonography and quantitative ONSD evaluation could help ophthalmologists directly observe structural abnormalities of the optic nerve, suspect DON development and make timely referrals. It would be particularly useful in settings where orbital imaging facilities (e.g., CT and MRI) were limited. Moreover, given the significant differences between DON and non-DON eyes, we speculated that there might be a decrease of ONSD in DON eyes following treatment. It is interesting to validate the relationship between ONSD and visual function, and to explore the predictive performance of ONSD in DON treatment through cohort studies.
Recent studies have shown that the ultrasonographic measurement of ONSD is a promising tool to predict raised ICP in emergency departments [13, 17]. ONSD changed in patients with optic neuropathy (DON, glaucoma [14], and acute neuritis [15]), which did not have intracranial hypertension. This suggests that using ONSD evaluation as an ICP in an emergency should be cautiously done, as patients might have concomitant optic neuropathies.
The main advantages of our study were the relatively large sample size and favorable inter-researcher agreement on ONSD measurement. The main limitation of this study arised from its cross-sectional design. This meant we were unable to determine the causal relationship between ONSD alteration and DON development. Another limitation was that we excluded a portion of TAO patients with severe strabismus, which might result in selection bias. Finally, the disease course of DON, which was whether patients had chronic or acute DON, was not considered. The majority of DON patients in our study had acute DON. The ONSD characteristics of chronic DON eyes needs to be examined further.
In conclusion, our study showed that the ultrasonographic measurement of ONSD was simple, reliable, and a low-cost method for evaluating the structural abnormalities of the optic nerve in DON patients. ONSD can be used to distinguish DON from non-DON eyes. Widening of the retrobulbar segment of the optic nerve can be an imaging marker of intraorbital optic nerve compression.
Summary
What was known before:
-
ONSD has been used as a quantitative indicator of translaminar pressure gradient for exploring the pathogenesis of glaucoma, and as an auxiliary parameter facilitating the diagnosis of acute optic neuritis.
-
However, ultrasonographic based ONSD changes have not yet been documented in TAO patients, especially in those with DON.
What this study adds:
-
An increase in ONSD is evident in DON eyes independent of clinical activity.
-
Ultrasound-based ONSD has sufficient ability to distinguish DON from non-DON eyes.
References
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38.
Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. 2017;12:111–21.
McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–8.
Saeed P, Tavakoli Rad S, Bisschop P. Dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg. 2018;34(4S Suppl 1):S60–S67.
Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Donnelly J, et al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: a prospective observational study. PLoS Med. 2017;14:e1002356.
Siaudvytyte L, Januleviciene I, Ragauskas A, Bartusis L, Siesky B, Harris A. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmol. 2015;93:9–15.
Lochner P, Leone MA, Coppo L, Nardone R, Zedde ML, Cantello R, et al. B-mode transorbital ultrasononography for the diagnosis of acute optic neuritis. A systematic review. Clin Neurophysiol. 2016;127:803–9.
Yee NP, Kashani S, Mailhot T, Omer T. More than meets the eye: point-of-care ultrasound diagnosis of acute optic neuritis in the emergency department. Am J Emerg Med. 2019;37:177 e171–177 e174.
Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–5.
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5:9–26.
Dubost C, Le Gouez A, Jouffroy V, Roger-Christoph S, Benhamou D, Mercier FJ, et al. Optic nerve sheath diameter used as ultrasonographic assessment of the incidence of raised intracranial pressure in preeclampsia: a pilot study. Anesthesiology. 2012;116:1066–71.
Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2018;44:1284–94.
Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68.
Jaggi GP, Miller NR, Flammer J, Weinreb RN, Remonda L, Killer HE. Optic nerve sheath diameter in normal-tension glaucoma patients. Br J Ophthalmol. 2012;96:53–56.
Lochner P, Cantello R, Brigo F, Coppo L, Nardone R, Tezzon F, et al. Transorbital sonography in acute optic neuritis: a case-control study. AJNR Am J Neuroradiol. 2014;35:2371–5.
Zhang T, Xiao W, Ye H, Chen R, Mao Y, Yang H. Peripapillary and macular vessel density in dysthyroid optic neuropathy: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci. 2019;60:1863–9.
Bauerle J, Schuchardt F, Schroeder L, Egger K, Weigel M, Harloff A. Reproducibility and accuracy of optic nerve sheath diameter assessment using ultrasound compared to magnetic resonance imaging. BMC Neurol. 2013;13:187.
Funding
The present work was supported by the National Natural Science Foundation of China (81600751, 81700875, 81470664, 81670887, and 81870689).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, X., Xiao, W., Ye, H. et al. Ultrasonographic measurement of the optic nerve sheath diameter in dysthyroid optic neuropathy. Eye 35, 568–574 (2021). https://doi.org/10.1038/s41433-020-0904-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0904-2
This article is cited by
-
Optical coherence tomography and shear wave elastography findings in Graves ophthalmopathy
International Ophthalmology (2024)