Abstract
Purpose
Juvenile onset primary open angle glaucoma (JOAG) is a rare disorder associated with high IOP and progressive optic neuropathy in patients diagnosed before the age of 40 years. While in some populations it has primarily an autosomal dominant pattern of inheritance, in others it occurs in a primarily sporadic form. The main aim of the study was to assess the relative prevalence of Myocilin (MYOC) mutations in familial versus sporadic cases of JOAG.
Methods
We screened 92 unrelated (sporadic) JOAG patients, and 22 affected families (70 affected members and 36 unaffected) for variations in the MYOC gene. We also analyzed the clinical features associated with these variations.
Results
Three coding sequence variants were identified as mutations causing JOAG. Four families segregated distinct mutations at Gly367Arg, and two families at Gln337Arg, while only two sporadic JOAG cases harbored MYOC mutations (Gly367Arg and Gln48His). The frequency of MYOC mutations in familial cases (27%) was significantly higher than in sporadic JOAG cases (2%); p = 0.001. A 90% penetrance for the Gly367Arg variant was seen by the age of 40 years in our patients. Characteristic allele signatures, indicative of specific founder effects, were not observed for the Gly367Arg mutation that was looked for in 12 patients among 2 geographically close families, which harbored this mutation.
Conclusion
Our data demonstrated that genetic screening for MYOC mutations should be focused toward cases with familial rather than sporadically occurring JOAG.
Similar content being viewed by others
Introduction
Juvenile onset open angle glaucoma (JOAG) is primary open angle glaucoma with an age of onset before 40 years. It is relatively uncommon with prevalence between 0.7 and 3.3% among glaucoma patients [1, 2]. These patients present with high intraocular pressure (IOP) and deep steep cupping of the optic nerve head and are likely to suffer great visual disability if left undiagnosed and untreated [3]. While it was considered to be inherited in an autosomal dominant fashion [4,5,6,7], recent studies have shown pedigrees with an autosomal recessive pattern as well as sporadic occurrence of the disease [8, 9]. In a previous study, we found that majority of JOAG patients do not have a familial association [10]. The prevalence of Myocilin (MYOC) mutation has been shown to be higher among JOAG patients than other types of primary glaucoma [11,12,13,14,15]. Apart from the fact that detection of MYOC mutations among patients is helpful in screening family members and thus being able to detect the disease at an earlier stage in some [16], recent experimental work has shown the efficacy of therapeutic targets specifically among Myocilin glaucoma [17]. Most of MYOC mutations however have been documented among families where many members were affected with glaucoma [4, 14]. However, there are two reports of MYOC de novo mutation occurring in sporadic JOAG [9, 18]. It is not established from current literature whether genetic screening of nonfamilial JOAG patients is advisable. Hence the important question arises, whether nonfamilial JOAG patients should be screened for MYOC mutations. In order to answer this question, it is important to assess the prevalence of MYOC mutations among familial and sporadic JOAG patients in the same population. In this study, we compared the prevalence of MYOC mutations among JOAG cases that were familial (with at least 2 other first degree family members other than the proband affected with glaucoma) with those, without any other family member having glaucoma (sporadic).
Methods
This study included JOAG patients who were being followed up at our center (Dr R.P. Centre for Ophthalmic Sciences, AIIMS, New Delhi; a tertiary eye care center in North India) and whose first degree relatives, above 10 years of age, were available for examination. Ophthalmic investigations and medical histories were recorded for all family members and patients enrolled for the study. These patients were evaluated by Senior glaucomatologists involved in their care. The study followed the tenets of Declaration of Helsinki and was approved by our Institutional Ethics committee. An informed consent was obtained from all participants in the study. A special consent for a genetic evaluation was obtained from all patients whose blood was withdrawn for analysis. Sporadic cases were defined as the ones in which no family member, other than the proband had glaucoma as determined by examining all first degree relatives (above the age of 10 years) over three generations. Familial cases were considered to be those who had at least two other first degree relatives, other than the proband, diagnosed with glaucoma.
JOAG was diagnosed as POAG occurring before the age of 40 years. The following criteria were used for ascertaining the presence of POAG amongst the probands: Increased IOP > 22 mmHg on at least two occasions in the presence of glaucomatous neuropathy in at least one eye ascertained using the Scanning laser ophthalmoscopy with or without visual field loss consistent with optic nerve damage along with wide open angles on gonioscopy in both eyes. Central corneal thickness (CCT) was assessed for all patients and IOP readings were corrected for the CCT. The optic nerve head topography was analyzed using a confocal scanning laser ophthalmoscope; Heidelberg Retinal Tomograph II (Heidelberg Engineering, Germany). The optic disc was considered to be glaucomatous in the presence of localized or generalized retinal nerve fiber layer defect with thinning of neuro-retinal rim or with the presence of a documented decrease in neuro-retinal rim. A glaucomatous scotoma on perimetry was considered in the presence of at least a cluster of three or more non edged points with a p < 5% sensitivity with at least one having a sensitivity that occurs in fewer than 1% population on pattern deviation probability plot, the points being in the expected location for glaucomatous defect, a pattern standard deviation that occurs in <5% of normal reliable fields or the glaucoma hemi field test indicating abnormality [19]. Ocular hypertensives (OHT) were patients defined as having documented increased IOP > 22 mmHg (corrected for the CCT) on more than two occasions in the absence of a glaucomatous optic neuropathy.
Exclusion criteria were those with history of steroid use, presence of any retinal or neurological pathology, evidence of secondary causes of raised IOP (pigment dispersion, pseudo exfoliation, or trauma), those with any pathology detected on gonioscopy such as angle recession, pigmentation of the angle greater than grade 3 or peripheral anterior synechiae, those with corneal diameter > 12 mm and those who had had any ocular surgery other than for glaucoma before presentation. Patients, none of whose first degree relatives were available for examination were excluded.
DNA preparation and Sanger sequencing
Five milliliter blood of the patients and family members was collected in ethylene diamine tetra acetate vial and genomic DNA was isolated from leukocytes by Miller’s salting out method [20]. All the three coding exons of MYOC were amplified by polymerase chain reaction (PCR) using primers flanking coding region described previously; with slight modification for exon2 primers (5′TGCCACCACATCCAGCTAAT3′F and CTCTGCTCCCAGGGAAGTTA3′R) [21]. The PCR amplifications were performed using 100 ng DNA in BioRad Thermal Cycler (Thermo Scientific, Waltham, MA). PCR cycle condition consisted of an initial denaturation step at 94 °C for 5 min followed by 34 cycles of denaturation at 95 °C for 30 s and 62 °C for 30 s annealing which was followed by extension at 72 °C for 25 s and final elongation at 72 °C for 5 min. Subsequently PCR products were diluted in five volumes of PB buffer (Qiagen, Mississauga ON), transferred on a Whattman GF/C filter plate, washed twice with 80% ethanol/20 mM Tris (pH 7.5) and eluted in 50 ml of water. A second PCR using forward primer was performed using the purified PCR product as template on Applied Biosystems Gene Amp PCR System 9700 machines to incorporate the sequencing dyes. Amplification conditions included 25 cycles of denaturation (95 °C for 10 s) and annealing (55 °C for 5 s), followed by one last step of elongation (59 °C for 2 min). PCR products were purified by the ABI ethanol–EDTA precipitation protocol, collected using a Beckman-Coulter Allegra 6R centrifuge, and resuspended in a 50% HiDi-formamide solution. Samples were then run on Applied Biosystems Prism 3700 DNA Analyzer automated sequencers. Sequence data were analyzed using the Unipro UGENE programs.
Haplotype analysis was performed in two pedigrees positive for Gly367Arg mutation. Genomic DNA of proband and family members was used for determining the haplotype. Haplotypes were derived by fragment analysis using two short tandem repeat (STR) polymorphic markers (D1S2815 and D1S2790) that closely flank the MYOC gene and an intragenic marker c.1020G > A (rs534806873). The forward primers of the STRs, D1S2815, and D1S2790 were labeled with HEX or FAM fluorophores at the 5′ end as described previously [5, 22]. The STRs PCR products were genotyped by automated electrophoresis in the ABI PRISM 3500 DNA Analyzer (Applied Biosystems, Foster City, CA, USA) and the base pairs was assessed using the Geneious version 10.0 software, followed by a comparison of the allele size of each STR between the two families.
Age-related penetrance of the mutations was calculated considering three ages: 20, 30, and 40 years, as a ratio of mutation-carrying subjects who were diagnosed less than the specified age, divided by the total number of people diagnosed less than the specified age and all mutation carriers older than this who were not diagnosed with the disease by that respective age [23].
Statistical analysis
Statistical Package for Social Sciences (SPSS ver.20 Chicago, IL, USA) was used for analysing the data. Significance of difference in age of onset and IOP among different groups of patients was determined by the Mann–Whitney U test where the data distribution did not follow the pattern of normality and an independent t test to compare normally distributed data. A Chi square was used to compare categorical variables and p value was considered significant for a p < 0.05.
Results
The study included 22 families and 92 sporadic JOAG patients of a North Indian cohort. The demographic and clinical details of the subjects are given in Table 1.
Of all the subjects (n = 106) examined among the 22 JOAG families, 70 (66%) were affected and 36 (34%) were unaffected. All the study subjects were included for screening of all three exons of MYOC gene. We found a heterozygous transition at c.1099G > A (p.Gly367Arg) in 20 affected members belonging to 4 JOAG families (Fig. 1). A pathogenic variant Gln337Arg in homozygous condition was also found in six patients among two other families (Fig. 2). Among the 92 sporadic JOAG cases, Gly367Arg mutation was observed in one patient (1.1%) and Gln48His variant in another JOAG patient (1.1%). However, the DNA of relatives of these patients was not available for screening these mutations among family members. Considering the probands in our study, 6 among familial (out of 22) and 2 among the sporadic (out of 92) harbored MYOC mutation (χ2 = 13.5; p < 0.001).
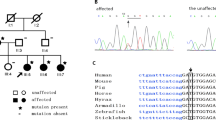
a Blood samples of subjects from A to I were available for the study. Those shaded in black were diseased (glaucoma/Ocular hypertension). Those denoted with the asterisk had the mutation. Age of disease onset is denoted for each in years [brackets]. b Chromatogram showing the heterozygous Gly367Arg mutation in exon 3, of MYOC.c.1099G > A caused an amino acid change from Glycine to Arginine at codon 367.
a Blood samples of subjects from A to E were available for the study. Those shaded in black were diseased (glaucoma/Ocular hypertension).Those denoted with the asterisk had the mutation. Age of disease onset is denoted for each in years [brackets]. b Chromatogram showing the homozygous Gln337Arg mutation in exon 3 of MYOCc.1010A > G caused an amino acid change from Glutamine to Arginine at codon 337.
Apart from the mutations, we detected three polymorphisms in the coding region, c.227G > A;p.Arg76Lys (rs2234926),c.366C > T;p.Gly122Gly (rs145354114), c.1020G > A; p.Glu340Glu (rs534806873) and one in intron two region IVS2 730 + 35G > A (rs2032555) Table 2. This (rs2032555) polymorphism was present in all of our sporadic patients except one who had wild genotype. Except for p.Glu340Glu that was observed in two familial patients, all polymorphisms were observed only among sporadic JOAG patients.
Among the familial JOAG (n = 22), there were no significant differences among the probands with myocilin mutation (n = 6) and without mutation (n = 16) for median age of onset (27 years versus 26 years, p = 0.92; Mann–Whitney U test) as well as the average highest untreated IOP (38.6 ± 6.1 mmHg versus 30.2 ± 7 mmHg; p = 0.12; independent t test).
Considering the Gly367Arg as the most commonly seen mutation in this study, we evaluated the phenotypic features of the diseased individuals carrying this mutation in the four families; the average age of onset (26.1 ± 7.8 years), the highest untreated IOP (43.6 ± 9.1 mmHg), the worst eye Mean Deviation (−21 ± 12.8 Db) and the worst eye vertical cup disc ratio (0.75 ± 0.2).
Age-related penetrance in relation to glaucoma/OHT of the Gly367Arg in our study group was found to be 75% (6/8) at 20 years, 85% (11/13) at 30 years and 91% (20/22) at 40 years of age. Two of the four families harboring the Gly367Arg mutation were found to belong from a similar geographic location (Azamgarh, Uttar Pradesh, India) within a 50-mile radius. To look for a founder effect of the Gly367Arg mutation among these two families we conducted a haplotype analysis. Haplotype analysis was based on the genotype data obtained from 12 members of 2 families for two STR polymorphic markers (D1S2815 and D1S2790) and an intragenic SNP marker c.1020G > A (Fig. 3). The allele sizes of STR markers in the affected members were (218, 222 bp) for D1S2815 and (240 and 242 bp) for D1S2790 (Fig. 3). In both families, the disease haplotype was different in terms of the allele size of the STRs suggesting that the mutation possibly occurred independently and the mutant gene in both the families might have originated from different ancestors.
Discussion
In a previous study, we reported a much higher prevalence of sporadic forms of JOAG in our population [10]. In the same study, a segregation analysis pointed to the fact that JOAG may not be a monogenic disease. Since MYOC has been shown to be the most widely prevalent gene mutation identified among JOAG patients, and since its prevalence has not been evaluated among the sporadic forms of JOAG, in this study we compared the prevalence of MYOC mutations in a well phenotyped, and a relatively large series of both familial and sporadic JOAG patients. Screening of the MYOC gene has been reported in different ethnicities which support presymptomatic molecular diagnosis in the respective pedigrees for specific genetic variations [24,25,26]. Genetic screening in select cases of familial JOAG has been shown to be a powerful tool to improve the diagnostic accuracy and determine best treatment methods [27]. Screening for genetic mutations in early onset glaucoma patients is important as it may determine their choices in many aspects of their future life [28]. However, screening all JOAG patients may be limited due to financial constraints among geographic locations where resources are limited along with the fact that genetic heterogeneity/incomplete penetrance/linkage disequilibrium can put a hindrance in identifying a cause and effect relationship [29,30,31]. This study found sporadic JOAG patients to have significantly lower prevalence of MYOC mutations in comparison to familial cases. Given the low prevalence of disease causing MYOC mutations among sporadically occurring JOAG patients, at least in this population, we would not consider screening for it. However, even the majority of familial JOAG cases lacked the MYOC mutation pointing toward other loci even among the autosomal dominant JOAG, that need to be investigated.
Nevertheless, the observed frequency of 27% in our familial JOAG was higher than the frequency observed in the largest study of 25 North American origin JOAG families with only 8% of them harboring MYOC mutations [32]. Shimuzu however, reported a higher prevalence of MYOC mutations (33%) in a smaller series (three out of nine) of Japanese families of JOAG patients [15].
Among our set of JOAG patients, the Gly367Arg mutation was most commonly detected mutation. Except one JOAG patient from Southern India [33], none of the other studied POAG/JOAG patients have been shown to carry the Gly367Arg mutation from this part of the world. Most studies have shown an association of Gly367Arg mutation with earlier onset of POAG [34,35,36]. The mean age of diagnosis in Gly367Arg carriers was found to be 34 years in Quebec, 36 years in Japanese, and 32 years in Indian JOAG patients [33, 37, 38]. The maximal IOP ranged from 30 to 50 mmHg [33, 34, 37] among patients with Gly367Arg mutation [36, 38]. In a German population, Gly367Arg was specific to familial JOAG patients as the mutation was completely absent in sporadic POAG patients and normal healthy controls [39]. Because of the higher prevalence and relatively high penetrance of Gly367Arg mutation among families, it is worthwhile screening all family members whenever a JOAG patient is found to carry this mutation. We did however observe this mutation in one (1.1%) of the sporadically occurring JOAG patients. We did not have the DNA of the family members of this patient to ascertain the occurrence of this mutation in other members of the family.
Gly367Arg is localized in the C-terminal part of the protein which is a major component of the extracellular matrix of the olfactomedin domain of MYOC that is linked to early onset, inherited forms of open angle glaucoma [13]. The wild type MYOC is normally secreted in to the trabecular extracellular matrix but recent evidence supports a toxic gain of function for MYOC mutations [13, 40, 41]. The mutations especially in the C-terminal part could interfere with the uptake or metabolism of protein, leading to misfolding and accumulation of protein in the endoplasmic reticulum and its impaired cellular trafficking thereby obstructing aqueous outflow [42, 43].
Our study suggests Gly367Arg mutation to be more commonly associated with JOAG in North Indian families. Few mutations are geographic or ethnicity specific; Cys433Arg mutation of exon 3 is related to JOAG in Brazilian families with autosomal dominant inheritance [26]. The other widely prevalent and recurrent MYOC pathogenic mutations; Pro370Leu and Gln368Stop with varying frequency/phenotype in POAG patients, were found to be absent in our series [5, 39, 44, 45]. Penetrance of Gly367Arg in our familial cases was 90% by 40 years of age which was higher than the earlier report in a Swiss family [34]. Even though MYOC mutations account for the most common genetic cause of autosomal dominant JOAG, all the mutations reported till now have shown incomplete penetrance [34, 36]. In our study, also we found two unaffected individuals with the Gly367Arg mutation. The unaffected individuals with the Gly367Arg mutation had no other genetic variations in MYOC coding exons. It is likely that these individuals could develop the disease at a later time in life and hence need to be closely monitored.
The other mutation, Gln48His unique to the Indian population, first described by Chakrabarti et al., who found it to be the most prevalent among their patients, was however identified in only one of our sporadic JOAG patient [46, 47]. Either the penetrance of this mutation is low as none of the family members of this case had glaucoma or it occurs as a de novo mutation. Sripriya et al. [47] described an adult onset POAG case with no family history of glaucoma harboring the Gln48His mutation. We did not have access to the DNA of the family members of this patient; hence it is difficult to say conclusively if it was a de novo mutation in our patient.
The Gln337Arg is another rare mutation that was detected in two families in our series and has only once been reported previously in a large JOAG family from Scotland. It is speculated that the mutation interferes with conformation of myocilin protein, thus being pathogenic [48].
The current study also identified commonly found polymorphisms observed in other populations. Glu340Glu (rs534806873) polymorphism was found in 18% among the familial group. The Arg76Lysrs (rs2234926) polymorphism was present in 39% of our sporadic JOAG patients as compared with the higher frequency among (73.2%) POAG patients from Eastern India and 25% in Southern India [44, 47]. This polymorphism was found in POAG patient population across the world with varied frequency ranging from 2 to 11% [49,50,51]. We found one sporadic JOAG patient with synonymous variation Gly122Gly (rs145354114). The Gly122Gly polymorphism observed in our study has been reported previously by Alward [4] as a non-disease causing polymorphism though Kangavalli reported this variation to be present in a POAG patient from Southern India but not in the healthy controls [4, 33]. The frequency of this polymorphism was 1–2% in all the reported POAG patient populations [5, 33, 50, 52]. The intronic variant (rs2032555) we found in our sporadic JOAG patients was reported earlier in glaucoma patients from Southern India, South Africa, and Iran [8, 21, 53] as a polymorphism. Although a functional role of this variation was not established, Pandaranayake et al. suggested that such polymorphisms in the MYOC genomic region which cause synonymous codon changes or that occurring in the intron regions can possibly lead to altered MYOC protein products through altered intron–exon splicing [53].
One of the limitations of the study was that we were unable to test the family members of the sporadic cases who also could have harbored the MYOC mutations and could possibly have developed glaucoma in future thus making these cases being actually familial type of JOAG. However, the strength of the study was a large number of unrelated sporadic cases and unrelated families of JOAG in a similar ethnic population that were available for screening for the MYOC gene. The low frequency of mutations in the most commonly related genes namely MYOC and CYP1B1 [54] among sporadic JOAG patients is suggestive of genetic heterogeneity at least in this population and further genetic studies should focus on this subset of patients to understand the role of other unidentified loci.
Summary
What was known before
-
Myocilin and Juvenile onset open angle glaucoma (JOAG): Myocilin mutations are associated with JOAG.
-
Prevalence of Myocilin mutations in JOAG: it was known that the prevalence of Myocilin mutations is higher among familial forms of JOAG and hence they need to be screened for Myocilin.
What this study adds
-
This study shows that among the sporadically occurring JOAG the prevalence of Myocilin mutations is low.
References
Das J, Bhomaj S, Chaudhuri Z, Sharma P, Negi A, Dasgupta A. Profile of glaucoma in a major eye hospital in north India. Indian J Ophthalmol. 2001;49:25–30.
Goldwyn R, Waltman SR, Becker B. Primary open-angle glaucoma in adolescents and young adults. Arch Ophthalmol 1970;84:579–82.
Gupta V, Ganesan VL, Kumar S, Chaurasia AK, Malhotra S, Gupta S. Visual disability among Juvenile open-angle glaucoma patients. J Glaucoma. 2018;27:e87–9.
Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med. 1998;338:1022–7.
Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905.
Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, et al. Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet. 1998;35:989–92.
Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, et al. Identification of a gene that causes primary open angle glaucoma. Science 1997;275:668–70.
Bayat B, Yazdani S, Alavi A, Chiani M, Chitsazian F, Tusi BK, et al. Contributions of MYOC and CYP1B1 mutations to JOAG. Mol Vis 2008;14:508–17.
Souzeau E, Burdon KP, Ridge B, Dubowsky A, Ruddle JB, Craig JE. A novel de novo Myocilin variant in a patient with sporadic juvenile open angle glaucoma. BMC Med Genet. 2016;17:30.
Gupta V, Somarajan BI, Gupta S, Chaurasia AK, Kumar S, Dutta P, et al. The inheritance of juvenile onset primary open angle glaucoma. Clin Genet. 2017;92:134–42.
Braghini CA, Neshich IAP, Neshich G, Soardi FC, de Mello MP, Costa VP, et al. New mutation in the myocilin gene segregates with juvenile-onset open-angle glaucoma in a Brazilian family. Gene. 2013;523:50–7.
Bruttini M, Longo I, Frezzotti P, Ciappetta R, Randazzo A, Orzalesi N, et al. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch Ophthalmol. 2003;121:1034–8.
Burns JN, Turnage KC, Walker CA, Lieberman RL. The stability of myocilin olfactomedin domain variants provides new insight into glaucoma as a protein misfolding disorder. Biochemistry. 2011;50:5824–33.
Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–61.
Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, et al. Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:165–77.
Souzeau E, Tram KH, Witney M, Ruddle JB, Graham SL, Healey PR, et al. Myocilin predictive genetic testing for primary open-angle glaucoma leads to early identification of at-risk individuals. Ophthalmology. 2017;124:303–9.
Zode GS, Bugge KE, Mohan K, Grozdanic SD, Peters JC, Koehn DR, et al. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Investig Ophthalmol Vis Sci. 2012;53:1557–65.
Kuchtey J, Chowdhury UR, Uptegraft CC, Fautsch MP, Kuchtey RW. A de novo MYOC mutation detected in juvenile open angle glaucoma associated with reduced myocilin protein in aqueous humor. Eur J Med Genet. 2013;56:292–6.
Anderson DR, Patella VM. Automated static perimetry; 2nd ed. St. Louis, Missouri: Mosby, 1999;152–3.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Whigham BT, Williams SE, Liu Y, Rautenbach RM, Carmichael TR, Wheeler J, et al. Myocilin mutations in black South Africans with POAG. Mol Vis. 2011;17:1064–9.
Marques AM, Ananina G, Costa VP, de Vasconcellos JPC, de Melo MB. Estimating the age of the p. Cys433Arg variant in the MYOC gene in patients with primary open-angle glaucoma. PLoS ONE. 2018;13:e0207409.
Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–11.
Lim P, Lichter PR, Higashi M, Downs CA, Richards JE. Septuagenarian’s phenotype leads to ascertainment of familial MYOC gene mutation. J Glaucoma 2003;12:98–103.
Mimivati Z, Nurliza K, Marini M, Liza-Sharmini A. Identification of MYOC gene mutation and polymorphism in a large Malay family with juvenile-onset open angle glaucoma. Mol Vis. 2014;20:714–23.
Povoa CA, Malta RF, Rezende Mde M, de Melo KF, Giannella-Neto D. Correlation between genotype and phenotype in primary open angle glaucoma of Brazilian families with mutations in exon 3 of the TIGR/MYOC gene. Arq Bras Oftalmol. 2006;69:289–97.
Miller MA, Fingert JH, Bettis DI. Genetics and genetic testing for glaucoma. Curr Opin Ophthalmol. 2017;28:133–8.
Khawaja AP, Viswanathan AC. Are we ready for genetic testing for primary open-angle glaucoma? Eye. 2018;32:877–83.
Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–9.
Gong G, Kosoko-Lasaki O, Haynatzki G, Roy Wilson M. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13:R91–102.
Tsui NBY, Cheng G, Chung T, Lam CWK, Yee A, Chung PKC, et al. Population-wide genetic risk prediction of complex diseases: a pilot feasibility study in Macau population for precision public healthcare planning. Sci Rep. 2018;8:1853.
Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, et al. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet. 1998;63:1549–52.
Kanagavalli J, Krishnadas SR, Pandaranayaka E, Krishnaswamy S, Sundaresan P. Evaluation and understanding of myocilin mutations in Indian primary open angle glaucoma patients. Mol Vis. 2003;9:606–14.
Iliev ME, Bodmer S, Gallati S, Lanz R, Sturmer J, Katsoulis K, et al. Glaucoma phenotype in a large Swiss pedigree with the myocilin Gly367Arg mutation. Eye. 2008;22:880–8.
Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S. Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am J Hum Genet. 1997;61:1202–4.
Yao YH, Wang YQ, Fang WF, Zhang L, Yang JH, Zhu YH. A recurrent G367R mutation in MYOC associated with juvenile open angle glaucoma in a large Chinese family. Int J Ophthalmol. 2018;11:369–74.
Faucher M, Anctil JL, Rodrigue MA, Duchesne A, Bergeron D, Blondeau P, et al. Founder TIGR/myocilin mutations for glaucoma in the Quebec population. Hum Mol Genet. 2002;11:2077–90.
Taniguchi F, Suzuki Y, Shirato S, Araie M. The Gly367Arg mutation in the myocilin gene causes adult-onset primary open-angle glaucoma. Jpn J Ophthalmol. 2000;44:445–8.
Michels-Rautenstrauss KG, Mardin CY, Budde WM, Liehr T, Polansky J, Nguyen T, et al. Juvenile open angle glaucoma: fine mapping of the TIGR gene to 1q24.3-q25.2 and mutation analysis. Hum Genet. 1998;102:103–6.
Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, et al. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 2015;24:2111–24.
Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, et al. Targeted disruption of the Myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–13.
Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600.
Sarfarazi M. Recent advances in molecular genetics of glaucomas. Hum Mol Genet. 1997;6:1667–77.
Mukhopadhyay A, Acharya M, Mukherjee S, Ray J, Choudhury S, Khan M, et al. Mutations in MYOC gene of Indian primary open angle glaucoma patients. Mol Vis 2002;8:442–8.
Mukhopadhyay A, Acharya M, Ray J, Khan M, Sarkar K, Banerjee AR, et al. Myocilin mutation 1109 C>T (Pro 370 Leu) is the most common gene defect causing early onset primary open angle glaucoma. Indian J Ophthalmol. 2003;51:279–81.
Chakrabarti S, Kaur K, Komatireddy S, Acharya M, Devi KR, Mukhopadhyay A, et al. Gln48His is the prevalent myocilin mutation in primary open angle and primary congenital glaucoma phenotypes in India. Mol Vis. 2005;11:111–3.
Sripriya S, Uthra S, Sangeetha R, George RJ, Hemamalini A, Paul PG, et al. Low frequency of myocilin mutations in Indian primary open-angle glaucoma patients. Clin Genet. 2004;65:333–7.
Stoilova D, Child A, Brice G, Crick RP, Fleck BW, Sarfarazi M. Identification of a new ‘TIGR’ mutation in a family with juvenile-onset primary open angle glaucoma. Ophthalmic Genet. 1997;18:109–18.
Mabuchi F, Yamagata Z, Kashiwagi K, Tang S, Iijima H, Tsukahara S. Analysis of myocilin gene mutations in Japanese patients with normal tension glaucoma and primary open-angle glaucoma. Clin Genet. 2001;59:263–8.
McDonald KK, Abramson K, Beltran MA, Ramirez MG, Alvarez M, Ventura A, et al. Myocilin and optineurin coding variants in Hispanics of Mexican descent with POAG. J Hum Genet. 2010;55:697–700.
Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, et al. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Investig Ophthalmol Vis Sci. 2002;43:3231–5.
Melki R, Idhajji A, Driouiche S, Hassani M, Boukabboucha A, Akhayat O, et al. Mutational analysis of the Myocilin gene in patients with primary open-angle glaucoma in Morocco. Ophthalmic Genet. 2003;24:153–60.
Pandaranayaka PJ, Prasanthi N, Kannabiran N, Rangachari K, Dhivya M, Krishnadas SR, et al. Polymorphisms in an intronic region of the myocilin gene associated with primary open-angle glaucoma—a possible role for alternate splicing. Mol Vis. 2010;16:2891–902.
Gupta V, Somarajan BI, Walia GK, Kaur J, Kumar S, Gupta S, et al. Role of CYP1B1, p.E229K and p.R368H mutations among 120 families with sporadic juvenile onset open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256:355–62.
Acknowledgements
This study was supported by funding from Directorate of Extramural Research and Intellectual Property Rights, Defence Research & Development Organization, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, V., Somarajan, B.I., Gupta, S. et al. The mutational spectrum of Myocilin gene among familial versus sporadic cases of Juvenile onset open angle glaucoma. Eye 35, 400–408 (2021). https://doi.org/10.1038/s41433-020-0850-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0850-z