Abstract
Background/objectives
Postoperative endophthalmitis is a rare, but serious complication of pars plana vitrectomy (PPV). Subconjunctival cefuroxime injection has been the traditional choice for post vitrectomy endophthalmitis prophylaxis. Its effectiveness and safety in this context are however poorly understood and cases of retinal toxicity have been reported. The traditional standard subconjunctival antibiotic prophylaxis has been superceded in cataract surgery by intracameral antibiotic prophylaxis.
Subjects/methods
The primary aim of this three centre non-randomised retrospective database cohort study of 7,532 PPV procedures was to identify the rate of endophthalmitis in cohorts of patients treated with intracameral or subconjunctival cefuroxime. A secondary aim was to estimate the achieved intraocular antibiotic concentrations of cefuroxime in eyes with intracameral versus subconjunctival administration using mathematical modelling.
Results
The overall incidence of postoperative endophthalmitis was 0.07% (5/7532). There were no cases of endophthalmitis in eyes receiving intracameral cefuroxime alone or in combination with subconjunctival cefuroxime (0/5586). Patients receiving subconjunctival cefuroxime alone had a higher incidence of endophthalmitis (0.22%, 4/1835), and there was one case of endophthalmitis in eyes not receiving any perioperative antibiotics (0.9%, 1/111). No cases of cefuroxime toxicity were identified. With subconjunctival cefuroxime, in the presence of a sclerotomy leak, we estimated the vitreous drug concentration to be higher than that for intracameral cefuroxime and potentially toxic.
Conclusions
Intracameral cefuroxime appears to be a safe and efficient choice for prophylaxis against endophthalmitis after PPV. Small eyes with intraocular tamponade seem to be at particular risk of drug toxicity if cefuroxime is administered via the subconjunctival route.
Similar content being viewed by others
Introduction
Postoperative endophthalmitis is a rare but potentially devastating complication following pars plana vitrectomy (PPV) [1]. By definition, it refers to the propagation of pathogenic organisms inside the eye postoperatively. As with other intraocular procedures, endophthalmitis following PPV is most commonly caused by gram-positive organisms, such as coagulase negative staphylococci [2]. Outcomes are generally poor, with a final visual acuity of light perception or worse in up to 33% of cases [1, 3, 4].
The Postoperative Endophthalmitis Study Group estimated the incidence of endophthalmitis after PPV to be 0.07% in a multicentre study of 12,216 patients in 1995 [1]. Since then, technology has rapidly developed with the advent of smaller gauge transconjunctival cannula based vitrectomy, and various groups have conducted retrospective studies, reporting an endophthalmitis incidence between 0.02 and 0.13% [4,5,6]. Despite initial concern, recent series have not identified an association between vitrectomy gauge and rates of postoperative endophthalmitis [4, 6, 7]. Risk factors for developing endophthalmitis after PPV include diabetes mellitus, vascular retinopathies, blepharitis, complex long surgery, leaking sclerotomies, absence of tamponade and postoperative hypotony [1, 4, 8,9,10]. Clearly some of these risks are non-modifiable, whereas others may be mitigated pre-operatively, and others are related to surgical technique.
Various methods are utilised to reduce the risk of endophthalmitis. Pre-operatively, this includes optimising general health, for example diabetic control, and treating co-existing ocular disease such as blepharitis. Peri-operatively, 5% povidone-iodine applied prior to commencing surgery has been shown to reduce the risk of bacterial endophthalmitis in patients undergoing cataract surgery, the precautionary principal makes it reasonable to presume the same to be true for PPV [11]. At the end of PPV surgery subconjunctival antibiotics have traditionally been used, although the evidence for choice of drug and their efficacy was shown to be limited in a retrospective case series of 18,886 patients [4, 8, 9, 12]. Indeed, it has been shown that some antibiotics may even be harmful. Subconjunctival gentamicin, for example, has been associated with a risk of retinal toxicity, macular infarction and severe visual loss [9, 13].
In general, cefuroxime, a second-generation cephalosporin, which exhibits broad-spectrum bactericidal activity against gram-positive and gram-negative organisms, has been used as prophylaxis in cataract and PPV surgery [14, 15]. It causes bacterial cell lysis by binding to penicillin binding protein sites to inhibit cell wall synthesis in a time and concentration dependent manner [16, 17]. The epidemiologic cut off values (Minimum Inhibitory Concentration separating a population into isolates with and without resistance to the drug) for cefuroxime for different organisms are as follows; Staphlococcus aureus (<4 mcg/ml), Streptococcus pneumoniae (<0.125 mcg/ml), Escherichia coli (<8 mcg/ml), Proteus mirabilis (<4 mcg/ml), Haemophilus influenzae (<2 mcg/ml) [17]. The aim of treatment is therefore to achieve concentrations of at least 8 mcg/ml.
Sub-conjunctival cefuroxime at doses of 62.5 mg/ml and 125 mg/ml for cataract surgery have been shown to result in aqueous humor concentrations of 12.33 mcg/ml and 20.23 mcg/ml, respectively, at 12–24 min post-injection [18]. Interestingly, a rabbit model showed that the vitreous cavity concentration of cefuroxime after subconjunctival administration was significantly lower than that achieved in the aqueous humor [19]. Once the drug is injected into the anterior chamber, it percolates into the vitreous cavity and equilibrates by diffusion and convection, dependent on the mesh size of the vitreous, drug molecular weight and net charge [20]. A vitrectomised eye is therefore likely to result in a faster distribution of drug in the vitreous cavity which is of importance in PPV endophthalmitis prophylaxis. In addition, if one considers that a subconjunctival drug may enter the vitreous cavity directly through a leaking sclerotomy, then the vitreous cavity concentration may be significantly higher, and inconsistent depending on the case and degree of leak.
A more consistent delivery of cefuroxime to achieve an accurate intraocular concentration, therefore, may be via the intracameral route which has both become standard and shown to offer superior postoperative endophthalmitis levels in cataract surgery [21]. Intracameral cefuroxime (Aprokam®, Laboratoires Thea, Clermont-Ferraud, France) is a single-use preparation which was approved by the European Medicines Agency for the antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery at a dose of 1 mg in 0.1 ml [22].
On the assumption that the mean anterior chamber volume is 0.17 ml, this intracameral dose achieves a concentration near 5.5 mg/ml which is considered more than that required for optimal bactericidal activity, will reduce as it diffuses into the posterior segment, and is significantly higher than would be achieved by subconjunctival administration [18, 23]. A retrospective study of 36,743 eyes found switching subconjunctival to intracameral cefuroxime resulted in a threefold decrease in endophthalmitis rates following phacoemulsification [24]. The concentration has been shown to be highly effective in reducing the rate of postoperative endophthalmitis following cataract surgery and is now standard care in most European units [14, 21].
Translating the safety and efficacy of intracameral cefuroxime to PPV causes debate, and there is minimal evidence in the literature apart from a case series of 152 combined phacovitrectomy procedures which found no endophthalmitis and no toxicity at a mean follow-up of 6.3 months [15]. There are no published reports of drug toxicity after intracameral cefuroxime in PPV. In vitro and animal studies have shown cefuroxime can exert direct toxic effects on retinal vascular endothelial cells [25]. Intracameral doses of 10–100 mg have been shown to be toxic in small case series and reports [26,27,28,29]. The total number of cases cited in these reports is 28 with a mean intracameral dose of 53.8 mg. Assuming an average intraocular volume of 7 ml, we estimate a toxic limit based on these cases to be 7.5 mg/ml.
The aims of this study were to:
-
1.
Compare the efficacy of intracameral cefuroxime with subconjunctival cefuroxime in preventing post PPV endophthalmitis.
-
2.
Mathematically estimate the vitreous concentrations of intracameral or subconjunctival cefuroxime in eyes undergoing PPV with or without internal tamponade.
Materials and methods
This was a retrospective, consecutive case series conducted in accordance with the principles of the Declaration of Helsinki. All vitreoretinal operations, including pars plana vitrectomies, performed at our institution under the care of 3 consultant vitreoretinal surgeons (THW, DAHL, RW) have been prospectively recorded on an electronic patient record (EPR) system (Vitreor, Axsys Technologies, Glasgow, UK) since October 1998.
An anonymised search was performed for all PPV operations undertaken between July 1997 and January 2020. The primary outcome was the incidence of postoperative presumed endophthalmitis on clinical examination findings. All patients had 5% povidone iodine instilled on the ocular surface, 10% povidone iodine to the skin and an aseptic placing of a surgical drape. At the conclusion of the case after sclerostomy closure, cefuroxime was either delivered using a 27-gauge needle to the subconjunctival space at a dose of 125 mg in 1 ml or via an angulated anterior chamber paracentesis intracamerally using a 30-gauge needle at a dose of 1 mg in 0.1 ml. All patients received a minimum of 2 weeks postoperative steroid and antibiotic eye drops.
The second part of the study was to mathematically calculate the potential doses of cefuroxime in the vitreous cavity depending on the degree of sclerotomy leak in eyes with or without ocular tamponade. We estimated the volume of tamponade to be 80% which takes into account residual vitreous, aqueous volume, lens volume and a small residual liquid layer in the vitreous cavity. A previous study has found a similar mean tamponade fill (78%) during macular hole surgery [30]. In order to estimate different eye volumes, we used a mathematical formula, based on axial length described by Nagra et al. [31]. This approximated the ocular volume in a hypermetrope, emmetrope and myope to be 4 ml, 7 ml and 10 ml, respectively. To estimate the cefuroxime concentration, we divided the dose of the injected drug by the ocular volume. In the absence of an alternative model we estimated a small and large sclerotomy leak to be 0.1 ml and 0.5 ml, respectively (which account for intraocular drug doses of 12.5 mg and 62.5 mg cefuroxime).
Results
There were 7532 PPVs performed during the study period. Of these, 2481 received 125 mg subconjunctival cefuroxime, and 5586 received 1 mg intracameral cefuroxime. 646 patients received antibiotics via both routes and 111 received no antibiotics. The incidence of postoperative endophthalmitis was 0.22% (4/1835) in the subconjunctival only group versus 0.00% (0/4940) in the intracameral only group [31]. There were no cases of endophthalmitis in patients receiving intracameral and subconjunctival cefuroxime together, and one case of endophthalmitis in patients who didn’t receive any antibiotics (0.9%, 1/111) There were no detected cases of toxicity secondary to cefuroxime in either the intracameral or subconjunctival group. The distribution of cases by vitrectomy gauge was as follows: 20-gauge, 71 PPVs; 23-gauge, 4450 PPVs; 25-gauge, 1398 PPVs; undocumented, 1613 PPVs.
Using the mathematical modelling of Nagra et al., most eyes have ocular volumes of between 4 and 10 ml dependent on their axial length [31]. Therefore, after intracameral cefuroxime (1 mg in 0.1 ml), the expected concentration in the vitreous cavity, with no tamponading agent, would be 0.3 mg/ml, 0.1 mg/ml and 0.1 mg/ml for an eye with an ocular volume of 4 ml, 7 ml and 10 ml, respectively.
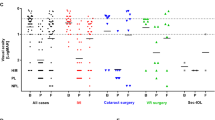
The estimated vitreous concentration following subconjunctival cefuroxime in 7 ml volume eyes with no tamponade agent and a small leak (0.1 ml), was 1.8 mg/ml, which increased to 9.0 mg/ml in eyes with a larger leak (0.5 ml). (Figure 1) Smaller eyes had higher concentrations, whereas larger eyes had lower concentrations of cefuroxime for the same volume of leak. Figure 1 shows the wide range of vitreous cavity concentrations obtained depending on antibiotic administration route and degree of sclerotomy leak. It also shows how these doses compare to a potentially toxic dose (7.5 mg/ml).
The vitreous concentration achieved with subconjunctival cefuroxime is significantly higher than intracameral cefuroxime. There is a linear relationship between degree of sclerotomy leak and vitreous cefuroxime concentration after subconjunctival delivery. Small volume eyes are at particular risk of toxicity, and normal volume eyes with a large leak also carry potential risk. The horizontal dashed line represents an estimated toxic dose level of 7.5 mg/ml. Cef = cefuroxime.
In eyes receiving an ocular tamponade, the predicted vitreous cavity cefuroxime concentration was higher due to the reduced aqueous volume present. With 1 mg intracameral administration, the vitreous concentration was 0.7 mg/ml in 7 ml volume eyes, increasing to 1.3 mg/ml in 4 ml eyes, and reducing to 0.5 mg/ml in 10 ml eyes. In 7 ml volume eyes receiving subconjunctival cefuroxime with a mild sclerotomy leak (0.1 ml), the vitreous concentration was 8.9 mg/ml, which increased to 44.6 mg/ml in eyes with a significantly leaky sclerotomy (0.5 ml). In 4 ml eyes, the concentration of cefuroxime was higher than the potentially toxic dose following subconjunctival administration with any degree of sclerotomy leak (e.g. 15.6 mg/ml with a 0.1 ml leak). (Figure 2).
In the presence of an intraocular tamponade, due to the reduced volume of distribution, the vitreous concentration of subconjunctival cefuroxime with any degree of sclerotomy leak is potentially toxic. The horizontal dashed line represents an estimated toxic dose level of 7.5 mg/ml. Cef = cefuroxime.
Discussion
We found the overall rate of endophthalmitis was low, measuring 0.07% (5/7532). This is in keeping with other database studies, which have found endophthalmitis rates between 0.02 and 0.13% [1, 4,5,6]. There were no cases of endophthalmitis in 5586 patients who received intracameral cefuroxime, compared to 0.22% (4/1835) in eyes which received subconjunctival cefuroxime. In addition to the superior efficacy, our predictions calculated that intracameral administration resulted in safer vitreous concentrations than subconjunctival administration of cefuroxime. As with any drug therapy, the priority is to achieve a therapeutic level with the lowest possible dose to minimise the chance of side effects. The therapeutic level to achieve bacteriocidal activity against the aforementioned organisms was 8 mcg/ml, which is far exceeded by intracameral cefuroxime in our study (0.1–0.3 mg/ml for an eye receiving no tamponade, and 0.5–1.3 mg/ml for an eye with tamponade). These concentrations are substantially lower than our calculated potential toxicity level (7.5 mg/ml). Therefore, our work gives support for intracameral cefuroxime being used as standard practice.
The risk of toxicity with cefuroxime is a complex issue. It has been shown that the viability of cultured endothelial cells can be significantly reduced when exposed to cefuroxime concentrations higher than 2.75 mg/ml [32]. This has potential implications for eyes undergoing phacoemulsification and the resultant damage to the endothelial cell layer from ultrasonic energy [33]. A small study of 90 patients found no difference in endothelial cell count between patients undergoing phacoemulsification with or without intracameral cefuroxime, however [34].
Regarding retinal toxicity, Koul et al. found no significant histopathological or electroretinography abnormalities after intravitreal injection of either 100 µg or 1 mg cefuroxime in rabbit eyes [35]. Another study on albino rabbit eyes found that 10 mg cefuroxime injected intravitreally caused structural and electroretinographic alterations [36]. Interestingly, Miyake et al. found an intravitreal injection of 2 µl of cefuroxime (2 mg/ml) resulted in direct retinal toxicity after 12 h and a strong inflammatory response with locally raised IL-8 and IL-1β levels that were dose-dependent [25]. Despite this, a large study has found 1 mg intracameral cefuroxime to be relatively safe in cataract surgery, with minimal toxicity reports at the recommended dose [21]. It is reassuring to know that most cases of retinal toxicity induced by intracameral cefuroxime have been with inadvertent overdose, although this reiterates the importance of using licensed products and being sure of the volume injected [26,27,28,29].
Intracameral cefuroxime is a consistent dose with intraocular concentrations, which are not strongly influenced by the integrity of the PPV sclerotomies. There is a wide range of practice regarding the closure of sclerotomy sites, with some receiving sutures and others not, and therefore it can be presumed that many cases have small leaks, which are often deemed clinically insignificant. Our data show that leaking sclerotomies have significant implications for patients receiving subconjunctival cefuroxime, who may therefore receive a potentially toxic dose, particularly when an ocular tamponade is also used.
We found that small eyes, with an estimated ocular volume of 4 ml, which have a tamponade are particularly at risk for cefuroxime toxicity after subconjunctival administration because any degree of sclerotomy leak results in a vitreous concentration over the predicted safe concentration of 7.69 mg/ml. In contrast, we found an intracameral dose of 1 mg in the same eyes would be unlikely to result in toxicity.
Limitations of our study include its retrospective nature and lack of randomisation. Cases receiving subconjunctival cefuroxime tended to be those earlier in the study period, and were more likely to be 20-gauge. Later cases tended to receive intracameral cefuroxime and had a higher proportion of 25-gauge instrumentation due to the timing of equipment change. Previous studies, however, have not shown that surgical PPV gauge affects the rate of endophthalmitis [4, 6, 7]. A further limitation on the concentration data in tamponade eyes is that the figures are based on a 80% tamponade fill. In reality, the degree of fill can vary, but is unlikely to be higher than this figure. Slight adjustments are unlikely to eliminate the potential risk of toxic doses for small eyes or those with tamponading agents and leaking sclerotomies. We estimated a toxic cefuroxime level from case reports in the literature, but this is likely to be an overestimate and therefore provides further support for intracameral instead of subconjunctival cefuroxime.
Our modelling suggests that a 1 mg intracameral dose will achieve therapeutic range concentrations of intraocular cefuroxime. We identified no cases of suspected ocular toxicity or endophthalmitis in a cohort control study of 5586 patients receiving this treatment. Our modelling has suggested that sub conjunctival injections of 125 mg may not achieve therapeutic intraocular doses and in the presence of scleraotomy leak can easily result in potentially toxic doses.
Summary
What was known before
-
Intracameral cefuroxime is routinely used in Europe as antibiotic prophylaxis in cataract surgery.
-
Pars plana vitrectomy antibiotic prophylaxis is less well understood and practice varies widely.
What this study adds
-
Intracameral cefuroxime appears to be a safe and efficient antibiotic choice for prophylaxis against endophthalmitis after PPV.
-
Subconjuntival cefuroxime produces a variable intraocular concentration of antibiotic which can potentially reach a toxic threshold dependent on ocular size, presence of tamponade and degree of sclerotomy leak.
References
Cohen SM, Flynn HW Jr, Murray TG, Smiddy WE. Endophthalmitis after pars plana vitrectomy. The Postvitrectomy Endophthalmitis Study Group. Ophthalmology. 1995;102:705–12.
Czajka MP, Byhr E, Olivestedt G, Olofsson EM. Endophthalmitis after small-gauge vitrectomy: a retrospective case series from Sweden. Acta Ophthalmol. 2016;94:829–35.
Park JC, Ramasamy B, Shaw S, Ling RH, Prasad S. A prospective and nationwide study investigating endophthalmitis following pars plana vitrectomy: clinical presentation, microbiology, management and outcome. Br J Ophthalmol. 2014;98:1080–6.
Scott IU, Flynn HW Jr, Acar N, Dev S, Shaikh S, Mittra RA, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2011;249:377–80.
Shi XY, Zhao HS, Wei WB. Analysis of post-operative endophthalmitis after pars plana vitrectomy: a 10-year experience at a single center. Chin Med J (Engl). 2013;126:2890–3.
Oshima Y, Kadonosono K, Yamaji H, Inoue M, Yoshida M, Kimura H, et al. Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Am J Ophthalmol. 2010;150:716–25. e1.
Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy causes and prevention. Ophthalmology. 2008;115:2215–20.
Eifrig CW, Scott IU, Flynn HW Jr, Smiddy WE, Newton J. Endophthalmitis after pars plana vitrectomy: incidence, causative organisms, and visual acuity outcomes. Am J Ophthalmol. 2004;138:799–802.
Kaiser RS, Prenner J, Scott IU, Brucker AJ, Flynn HW Jr, Williams GA, et al. The Microsurgical Safety Task Force: evolving guidelines for minimizing the risk of endophthalmitis associated with microincisional vitrectomy surgery. Retina. 2010;30:692–9.
Bhende M, Raman R, Jain M, Shah PK, Sharma T, Gopal L, et al. Incidence, microbiology, and outcomes of endophthalmitis after 111,876 pars plana vitrectomies at a single, tertiary eye care hospital. PLoS ONE. 2018;13:e0191173.
Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109:13–24.
Weiss SJ, Adam MK, Gao X, Obeid A, Sivalingam A, Fineman MS, et al. ENDOPHTHALMITIS AFTER PARS PLANA VITRECTOMY: efficacy of Intraoperative Subconjunctival Antibiotics. Retina. 2018;38:1848–55.
Cardascia N, Boscia F, Furino C, Sborgia L. Gentamicin-induced macular infarction in transconjunctival sutureless 25-gauge vitrectomy. Int Ophthalmol. 2008;28:383–5.
Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2017;2:CD006364.
Besozzi G, Di Salvatore A, Cardillo D, Finzi A, Pinackatt JS, Baldi A, et al. Intracameral cefuroxime in combined pars plana vitrectomy and phacoemulsification: a study of safety. Clin Ophthalmol. 2018;12:1567–70.
Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions. https://www.escrs.org/downloads/Endophthalmitis-Guidelines.pdf 2013 (Accessed 25 April 2020).
Compendium Em. Aprokam 50 mg powder for solution for injection: summary of product characteristics. France: Laboratoires Thea; 2012.
Jenkins CD, Tuft SJ, Sheraidah G, McHugh DA, Buckley RJ. Comparative intraocular penetration of topical and injected cefuroxime. Br J Ophthalmol. 1996;80:685–8.
Koul S, Philipson A, Philipson BT, Kock E, Nylen P. Intraocular levels of cefuroxime in uninflamed rabbit eyes. Acta Ophthalmol (Copenh). 1990;68:455–65.
Varela-Fernandez R, Diaz-Tome V, Luaces-Rodriguez A, Conde-Penedo A, Garcia-Otero X, Luzardo-Alvarez A, et al. Drug Delivery to the Posterior Segment of the Eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12:269.
Endophthalmitis Study Group ESoC, Refractive S. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88.
European Medicines Agency. List of nationally authorised medicinal products. Available at https://www.ema.europa.eu/en/documents/psusa/cefuroxime-sodium-intracameral-use-list-nationally-authorised-medicinal-products-psusa/00010206/201505_en.pdf. Accessed 25 April 2020.
Labiris G, Gkika M, Katsanos A, Fanariotis M, Alvanos E, Kozobolis V. Anterior chamber volume measurements with Visante optical coherence tomography and Pentacam: repeatability and level of agreement. Clin Exp Ophthalmol. 2009;37:772–4.
Yu-Wai-Man P, Morgan SJ, Hildreth AJ, Steel DH, Allen D. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:447–51.
Miyake H, Miyazaki D, Shimizu Y, Sasaki SI, Baba T, Inoue Y, et al. Toxicities of and inflammatory responses to moxifloxacin, cefuroxime, and vancomycin on retinal vascular cells. Sci Rep. 2019;9:9745.
Ciftci S, Ciftci L, Dag U. Hemorrhagic retinal infarction due to inadvertent overdose of cefuroxime in cases of complicated cataract surgery: retrospective case series. Am J Ophthalmol. 2014;157:421–5. e2.
Qureshi F, Clark D. Macular infarction after inadvertent intracameral cefuroxime. J Cataract Refract Surg. 2011;37:1168–9.
Delyfer MN, Rougier MB, Leoni S, Zhang Q, Dalbon F, Colin J, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011;37:271–8.
Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmol. 2012;90:e153–4.
Alberti M, la Cour M. NONSUPINE POSITIONING IN MACULAR HOLE SURGERY: a Noninferiority Randomized Clinical Trial. Retina. 2016;36:2072–9.
Nagra M, Gilmartin B, Logan NS. Estimation of ocular volume from axial length. Br J Ophthalmol. 2014;98:1697–701.
Yoeruek E, Spitzer MS, Saygili O, Tatar O, Biedermann T, Yoeruek E, et al. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34:2139–45.
Zhang JY, Feng YF, Cai JQ. Phacoemulsification versus manual small-incision cataract surgery for age-related cataract: meta-analysis of randomized controlled trials. Clin Exp Ophthalmol. 2013;41:379–86.
Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28:977–81.
Koul S, Philipson A, Philipson BT, Arvidson S. Intraocular levels of cefuroxime in inflamed rabbit eyes. Eur J Ophthalmol. 1993;3:61–5.
Shahar J, Zemel E, Perlman I, Loewenstein A. Physiological and toxicological effects of cefuroxime on the albino rabbit retina. Invest Ophthalmol Vis Sci. 2012;53:906–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neffendorf, J.E., Kumaran, N., Sandinha, T. et al. Safety of intracameral cefuroxime in pars plana vitrectomy. Eye 35, 2601–2606 (2021). https://doi.org/10.1038/s41433-020-01303-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01303-1