Abstract
Background/objectives
To report clinical outcomes of modified Collaborative Ocular Melanoma Study IRIS (COMS IRIS) plaques for treatment of iris, iridociliary, and ciliary body melanoma.
Subjects/methods
Retrospective, single-centre cohort study of iris melanoma treated with COMS IRIS plaque radiotherapy from July 26, 2010 to October 15, 2018. Medical records were reviewed for demographics, tumour features, treatment parameters, and clinical outcomes.
Results
There were 22 cases, diagnosed at mean age of 59 years (median 65, range 21–85 years) with female sex in 14 (64%). Presenting tumour features included Snellen visual acuity (VA) ≥ 20/40 in 18 (82%) cases, mean largest tumour basal diameter 4.7 mm (median 3.9, range 2.3–13.8 mm) and thickness 1.7 mm (median 1.6 mm, range 0.8–3.9 mm), iris stromal seeding in 3 (14%) cases, angle seeding in 16 (73%), and ciliary body involvement in 13 (59%). After mean follow-up of 51 months (median 44, range 4–113 months), Snellen VA was ≥20/40 in 14 (64%) cases, with local tumour recurrence in 2 (9%), and enucleation in 2 (9%). The 3-year Kaplan–Meier estimated risk of local tumour recurrence was 7%. The most common radiation side effects were cataract in 17 (77%) patients and dry eye in 5 (23%). Systemic metastasis occurred in no cases, and 1 (5%) non-melanoma-related death due to natural causes was observed at last follow-up.
Conclusions
COMS IRIS plaques are effective for treatment of iris, iridociliary, and ciliary body melanoma with modest VA outcomes and low frequency of local tumour recurrence, enucleation, radiation side effects, and systemic metastasis.
Similar content being viewed by others
Introduction
Iris melanoma is the least common subtype of uveal melanoma, accounting for ~3–4% of all uveal melanoma cases [1, 2]. In contrast to other forms of uveal melanoma, iris melanoma tends to occur in younger-aged and middle-aged adults, prognosis is typically more favourable due to less aggressive cytological features, and metastasis is estimated to occur in only 3–10% of patients after 5 years follow-up [3,4,5,6]. Plaque radiotherapy is a standard of care treatment for iris melanoma that achieves high rates of local tumour control from 92% to 100% with low risk for metastasis of 0–3% [7,8,9,10,11]. However, radiation side effects such as limbal stem cell deficiency, cataract, and glaucoma may limit visual acuity (VA) outcomes [8, 12, 13].
At our institution, modified Collaborative Ocular Melanoma Study plaques (COMS IRIS plaques) have been used for treatment of iris, iridociliary, and ciliary body melanoma over the past decade. COMS IRIS plaques are based on the 22 millimetre (mm) dome-shaped traditional COMS plaques with a 10 mm diameter hole in the centre and inner and outer collimating lips. Arc segments of the spherical shell come in three shapes: 180° arc (Iris-180), 270° arc (Iris-270), and 360° arc (Iris-360) (Fig. 1). By matching arc length to tumour extent, the theoretical advantage of COMS IRIS plaques is reduction of radiation exposure to structures outside the intended treatment area. We previously demonstrated reduced radiation dose to critical structures, such as the cornea, with the modified plaques, but have not yet described detailed patient outcomes [14]. Herein, we investigate the clinical outcomes of iris, iridociliary, and ciliary body melanoma treated with COMS IRIS plaque radiotherapy at our institution.
Seed diagrams modified based on previously reported figures [24]. A Traditional, round COMS plaque with 22 mm diameter. B 180° plaque (Iris-180). C 270° plaque (Iris-270). D 360° plaque (Iris-360).
Subjects and methods
We performed a retrospective review of all patients diagnosed with iris, iridociliary, and ciliary body melanoma and treated with COMS IRIS plaque radiotherapy as primary therapy at Mayo Clinic in Rochester, Minnesota from July 26, 2010 to October 15, 2018. Patients were excluded if they received treatment with other primary therapy prior to plaque radiotherapy, such as iridectomy or iridocyclectomy, or if they had <3 months of follow-up post-radiotherapy. This study was compliant with the Health Insurance and Portability and Accountability Act, was deemed exempt by the Mayo Clinic Institutional Review Board (IRB), and adhered to the tenets of the Declaration of Helsinki.
Demographic data, clinical features at presentation, radiotherapy treatment data, and clinical outcomes were reviewed. Demographic data included age, race, sex, involved eye, iris colour, medical/ocular history, and occupation. Clinical features at presentation included Snellen VA, intraocular pressure (IOP), pre-radiotherapy glaucoma medications, tumour diameter, tumour thickness, anatomic location, shape, elevation, surface, configuration, colour, associated features, tumour stage, cytology, and cytogenetics. Staging was reported based on the American Joint Commission on Cancer (AJCC) 8th edition staging criteria for iris, iridociliary, and ciliary body melanoma [15]. Treatment parameters included plaque shape (Iris-180, Iris-270, Iris-360), prescription dose, prescription depth, treatment duration, total source strength, total radiation dose to key anatomic structures, and radiation dose rate. Clinical outcomes included Snellen VA, IOP, tumour diameter and thickness, local tumour recurrence, enucleation, radiation side effects, metastasis, and death. Radiation side effects included dry eye, keratopathy, limbal stem cell deficiency, corneal melt, cataract, neovascularization of the iris (NVI), radiation glaucoma, neovascular glaucoma (NVG), scleral thinning, new or worsening extraocular extension, vitreous haemorrhage, rhegmatogenous retinal detachment, radiation maculopathy, radiation retinopathy (non-proliferative and proliferative), radiation papillopathy, neovascularization of the disc, neovascularization elsewhere, and retinal vessel occlusions. Data were collected from the electronic medical records through June 1, 2020. Statistical analysis was performed using Microsoft Excel 2010 (Microsoft Corporation; Redmond, WA, USA) for descriptive statistics. Spearman’s Rho analysis was conducted to assess the association between dosimetry and local tumour recurrence, and Kaplan–Meier risk for local tumour recurrence was calculated using SPSS Statistics Software Version 22 (IBM; Armonk, NY, USA).
The radiation doses to the tumour apex, prescription point, and ocular structures were calculated using published COMS IRIS plaque Monte Carlo Dosimetry [14]. The X, Y, Z coordinates, in the eye coordinate system, for the cornea (11.3, 0, 0), lens (7.7, 0, 0), macula (−11. 3, 0, 0), and optic disc (−10.6, ±4, 0) were obtained from Thomson et al. [14]. The outer ocular surface was defined as the location in which the Silastic insert contacts the eye. The sclera surface was defined as the distance of 1 mm from the outer ocular surface in a direction towards the centre of the eye. The heterogeneity-corrected doses to the points of interest were obtained by applying published correction factors to homogeneous dose calculations performed using TG43 with a line source approximation [16]. COMS IRIS plaques were loaded according to the diagrams in Fig. 1 using iodine-125 model 2301 seeds, and then structure-specific heterogeneity–homogeneity correction factors from Thomson et al. were applied. The correction factors for prescription depth, tumour surface, opposite retina, and outer ocular surface were presumed to equal their values as tabulated for a specific depth (e.g. 2 mm prescription depth) by Thomson et al. since correction factors varied by only a few percent over a 2 cm range of depths.
Results
There were 22 eyes of 22 patients diagnosed with iris, iridociliary, or ciliary body melanoma and treated with primary plaque radiotherapy with COMS IRIS plaques from July 26, 2010 to October 15, 2018. Patient demographics and clinical features at initial presentation are illustrated in Table 1. The mean age at presentation was 59 years (median 65, range 21–85 years). There were 21 (96%) patients who were white and 14 (64%) who were female. Ocular involvement was unilateral in all cases. The most common iris colour was blue in 13 (59%) cases. A positive past medical history of cutaneous melanoma was observed in 1 (5%) patient, while 2 (9%) had a positive family history of cutaneous melanoma. There was 1 (5%) case with ocular melanocytosis. No patients reported having an occupation as an arc welder, electrician, or x-ray technician.
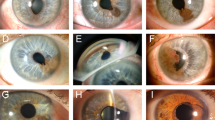
The mean Snellen VA at initial presentation was 20/30 (median 20/28, range 20/20–20/70), with Snellen VA ≥ 20/40 in 18 (82%) affected eyes. Mean IOP was 15 mmHg (median 15, range 10–22 mmHg), and 2 (9%) patients were on IOP lowering medication prior to radiotherapy. The mean largest tumour basal diameter was 4.7 mm (median 3.9, range 2.3–13.8 mm), mean tumour thickness was 1.7 mm (median 1.6, range 0.8–3.9 mm), and mean number of tumour clock hours was 2 (median 2, range 1–6). The most commonly affected iris quadrant was inferior in 14 (64%) patients, while the most frequent anatomic location of the tumour epicentre was midzonal in 9 (41%). In 2 (9%) patients, the tumour epicentre was in the ciliary body, and the posterior tumour margin involved the ciliary body in 13 (59%) cases. The most common tumour features were discrete tumour margins in 17 (77%) cases, minimal (flat) elevation in 11 (50%), smooth surface in 21 (96%), oblong configuration in 11 (50%), and melanotic pigmentation in 17 (77%). There were 3 (14%) cases with iris stromal seeding, and 16 (73%) with angle seeding. The most common AJCC Tumour (T) stage was T2a in 13 (59%) cases, followed by T1a in 8 (36%) cases. Cytology was collected for 4 (18%) patients, all of which demonstrated spindle cell predominance.
The treatment features of plaque radiotherapy are described in Table 2. There were 16 (73%) patients who were treated with Iris-180 plaques, 4 (18%) with Iris-270 plaques, and 2 (9%) with Iris-360 plaques. All patients were treated with iodine-125 model 2301 radioisotopes and a planned prescription dose of 85.0 Gray (Gy). The mean prescription depth was 5 mm (median 5, range 3–6), and mean treatment duration was 95 h (median 94, range 94–120 h). The mean total radiation dose to the tumour apex was 135.5 Gy (median 131.1, range 97.0–199.4 Gy), and the mean radiation dose rate to the tumour apex was 1.5 Gy/h (median 1.4, range 1.1–2.2 Gy/h).
The clinical outcomes after plaque radiotherapy are demonstrated in Table 3. From plaque radiotherapy to last follow-up visit, the mean follow-up time was 51 months (median 44, range 4–113 months). At last follow-up, 14 (64%) eyes had VA ≥20/40, and the mean number of lines of Snellen VA lost was 1 (median 0, range 0–8). Mean IOP at last follow-up was 18 mmHg (median 16, range 7–36 mmHg), and 11 (50%) patients required IOP lowering therapy during follow-up. The mean tumour basal diameter was 4.1 mm (median 4.0, range 1.0–7.0 mm) and mean tumour thickness was 1.4 mm (median 1.1, range 0.8–3.2 mm). Local tumour recurrence was observed in 2 (9%) cases, which occurred after 15 and 69 months post-radiotherapy; enucleation was required in both cases. The 3-year Kaplan–Meier estimated risk for local tumour recurrence was 7%. Lower radiation dose to tumour apex was correlated with increased risk for local tumour recurrence by Spearman’s rho analysis (p = 0.04). The most common radiation side effect was development or progression of cataract in 17 (77%) patients, diagnosed after a mean follow-up of 19 months (median 19, range 4–45 months) after plaque placement. There were 13 (59%) patients who underwent cataract surgery in the plaque-irradiated eye after a mean follow-up of 26 months post-radiotherapy (median 23, range 4–44 months). Other observed complications included dry eye in 5 (22.7%) cases, NVI in 1 (4.5%), and NVG in 1 (4.5%). There were no cases with keratopathy, limbal stem cell deficiency, corneal melt, radiation glaucoma, scleral thinning, new or worsening extraocular extension, vitreous haemorrhage, rhegmatogenous retinal detachment, radiation maculopathy, radiation retinopathy (non-proliferative and proliferative), radiation papillopathy, neovascularization of the disc, neovascularization elsewhere, or retinal vessel occlusion. Systemic metastasis was not observed in any patient, and 1 (5%) death due to natural causes (unrelated to iris melanoma) was noted at last follow-up.
Discussion
Plaque radiotherapy is a standard of care treatment for iris melanoma, yet radiation-related complications such as limbal stem cell loss, NVI, and radiation glaucoma are significant vision-threatening comorbidities [17]. In an attempt to decrease radiation outside the intended treatment area, we have utilized COMS IRIS plaques for treatment of iris, iridociliary, and ciliary body melanoma. A previous dosimetry study by our institution suggested COMS IRIS plaques may theoretically reduce radiation outside the treatment area [14], but long-term patient outcomes were unknown. In this single centre retrospective cohort study, we investigated the clinical outcomes of patients treated with COMS IRIS plaques for iris, iridociliary, and ciliary body melanoma over an 8-year period.
Traditional COMS plaques are dome-shaped with diameters ranging from 10 to 22 mm, Modulay (gold alloy) backings (0.5 mm thick), an outer radius of curvature of 15.05 mm, and a 2.7 mm outer collimating lip (0.5 mm thick) (Fig. 1). The concave surface of the plaque contains a Silastic (silicone polymer) seed carrier insert for brachytherapy seeds. The Silastic has an inner radius of curvature of 12.3 mm to match the curvature of the outer ocular surface. The Silastic insert secures the radioactive seeds 1 mm from the Silastic’s concave surface, and seeds are arranged in concentric circles around the plaque’s central axis. Although traditional COMS plaques are effective at delivering radiotherapy in iris melanoma, the uninterrupted dome design may lead to treatment of a wider field than required and radiation-related side effects can lead to chronic discomfort and vision loss. We previously reported the design and dosimetry of COMS IRIS plaques to decrease radiation exposure to structures outside the intended treatment area [14]. COMS IRIS plaques are segmental arcs of the 22 mm traditional dome-shaped COMS plaque in three shapes: Iris-180, Iris-270, and Iris-360. The COMS IRIS plaque design includes a 10 mm “hole” in the plaque centre surrounded by a 0.5 mm cylindrical segment of Modulay, inner and outer collimating lips, and a 0.5 mm-thick Modulay segment that connects the inner and outer collimating lips. COMS IRIS plaques theoretically reduce radiation dose outside the treatment area as demonstrated by Thomson and colleagues in a dosimetry study utilizing Monte Carlo simulations [14]. For non-circumferential tumours, matching plaque arc length to tumour extent reduces radiation to unaffected underlying structures. The centre hole and inner lip mitigate radiation-related injuries to the cornea and sclera, such as keratitis, erosions, thinning, and necrosis. The outer collimating lip and Modulay segment connecting the outer and inner lips attenuate radiation to surrounding structures such as the fellow eye.
Prior studies utilizing both traditional, round COMS plaques and uniquely shaped plaques have demonstrated excellent clinical outcomes for iris melanoma plaque radiotherapy, with local tumour control rates from 92% to 100%, enucleation rates from 0% to 13%, and metastasis rates from 0 to 5% [7,8,9,10,11, 18,19,20,21] (Table 4). Common radiation-related complications following plaque radiotherapy included decreased or poor VA in 5–75%, cataract in 36–73% of irradiated eyes, glaucoma in 3–33%, and corneal injury such as keratitis in up to 25% [7,8,9,10,11, 18, 19]. Long-term radiotherapy-associated corneal and scleral injuries rarely occur [7]. Shields et al. in a study of 144 patients utilizing round, notched, boomerang, doughnut, and custom-designed plaques, noted that recurrence risk increased with lower percentage of plaque coverage of the cornea (hazard ratio: 1.52, 95% confidence interval: 1.13–2.04, p = 0.006), with mean corneal plaque coverage of 30% for patients with recurrence and 63.7% for those with no recurrence. Other reported cases of specialized iris plaques include novel dome-shaped plaques with a smaller curvature radius to avoid plaque–corneal contact in two cases by Liu et al. [22], and modified Silastic inserts to improve plaque–cornea conformance in one case by Scanderbeg et al. [23].
Consistent with the literature, COMS IRIS plaques for the treatment of iris melanoma in the present study demonstrated a low frequency of local tumour recurrence in 9% of cases (7% 3-year Kaplan–Meier risk), enucleation in 9%, and systemic metastasis in 0% over a mean follow-up of 51 months. At last follow-up, poor VA (≤20/200) was observed in 14% of patients, cataract in 77%, NVI in 5%, and NVG in 5%. The local tumour control rate in this report was similar to that of the largest investigation of 144 patients by Shields and colleagues: 9% at mean 51 months follow-up compared to 7.8% at mean 40 months follow-up, respectively [9]. Local tumour recurrence in this study was associated with decreased radiation dose to the tumour apex, which could also be related to lower radiation dose to the tumour periphery, as the dosimetry of the tumour apex and periphery are correlated. Although our cohort included only 22 patients, this correlation could suggest that the minimum prescription depth of COMS IRIS plaques may differ from that of traditional COMS plaques, potentially due to the unique seed arrangement of COMS IRIS plaques or proximity of the plaque lip to the margins. With regard to plaque-related complications, there were no corneal complications, such as keratopathy, limbal stem cell deficiency, and corneal melt, potentially due to decreased segmental arc plaque–corneal contact. This study also supports previous findings that plaque radiotherapy for iris melanoma does not cause radiation-related posterior segment complications like radiation maculopathy or optic neuropathy [11]. We propose our COMS IRIS plaques as another innovation in iris melanoma radiotherapy given their potential to decrease radiation outside the treatment area and low risk of irreversible radiation-related side effects in this study.
There are several limitations of this study to consider, including a retrospective study design with variable follow-up pattern and duration. Patients without complications may have been more likely to follow-up with their local ophthalmologist, while those with complications might have followed up with their tertiary referral centre-based ocular oncologist, potentially inflating the number of complications reported in this study. This limitation was not exclusive to our study, as previous studies were also referral centre-based. Furthermore, the study cohort was small, as expected given the rarity of iris melanoma. Thus, some infrequent complications were not seen in any of our study patients, while a larger cohort might better detect the frequency of such events. Larger comparative trials are needed to elucidate differences in clinical outcomes and complication rates between traditional and COMS IRIS plaques.
In summary, COMS IRIS plaques may reduce radiation dose to critical ocular structures outside the treatment area in the management of iris, iridociliary, and ciliary body melanoma. In this cohort of 22 patients with a mean follow-up of over 4 years, we observed modest VA outcomes, low frequencies of local tumour recurrence and enucleation, and no cases of metastatic involvement. New development or progression of cataract was common but easily managed with cataract extraction. Vision-threatening radiation side effects such as NVI and NVG were rare. There were no corneal complications of keratopathy, limbal stem cell deficiency, or corneal melt, and no posterior segment complications of vitreous haemorrhage, retinal detachment, radiation maculopathy, radiation retinopathy, or radiation papillopathy. These findings suggest radiotherapy with COMS IRIS plaques is safe and effective for treatment of iris melanoma.
Summary
What was known before
-
Plaque radiotherapy is a standard of care treatment for iris melanoma that achieves high rates of local control.
-
Radiation side effects such as limbal stem cell deficiency, cataract, and glaucoma may limit visual acuity outcomes.
What this study adds
-
Among 22 patients with iris, iridociliary, and ciliary body melanoma treated with plaque radiotherapy, segmental arcs of traditional round COMS plaques demonstrated low frequency of recurrence or enucleation, with no metastasis over 4 years follow-up.
-
New development or progression of cataract was common but easily managed with cataract extraction. Vision-threatening radiation side effects such as neovascularization of the iris and neovascular glaucoma were rare.
-
Modified COMS IRIS plaques may reduce radiation dose to critical ocular structures outside the treatment area in the management of iris, iridociliary, and ciliary body melanoma.
References
Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012;32:1363–72.
Jensen O. Malignant melanomas of the human uvea: recent follow‐up of cases in Denmark, 1943–1952. Acta Ophthalmol. 1970;48:1113–28.
Shields CL, Kaliki S, Shah SU, Luo W, Furuta M, Shields JA. Iris melanoma: Features and prognosis in 317 children and adults. J AAPOS. 2012;16:10–6.
Singh AD, Damato BE. Iris melanoma. Clinical Ophthalmic Oncology: Springer; 2019. p. 155–84.
Shields CL, Shields JA, Materin M, Gershenbaum E, Singh AD, Smith A. Iris melanoma: risk factors for metastasis in 169 consecutive patients. Ophthalmology 2001;108:172–8.
Khan S, Finger PT, Yu G-P, Razzaq L, Jager MJ, De Keizer RJ, et al. Clinical and pathologic characteristics of biopsy-proven iris melanoma: a multicenter international study. Arch Ophthalmol. 2012;130:57–64.
Fernandes BF, Krema H, Fulda E, Pavlin CJ, Payne DG, McGowan HD, et al. Management of iris melanomas with 125Iodine plaque radiotherapy. Am J Ophthalmol. 2010;149:70–6. e2.
Razzaq L, Keunen JEE, Schalij-Delfos NE, Creutzberg CL, Ketelaars M, de Keizer RJW. Ruthenium plaque radiation therapy for iris and iridociliary melanomas. Acta Ophthalmol. 2012;90:291–6.
Shields CL, Shah SU, Bianciotto CG, Emrich J, Komarnicky L, Shields JA. Iris melanoma management with iodine-125 plaque radiotherapy in 144 patients: impact of melanoma-related glaucoma on outcomes. Ophthalmology 2013;120:55–61.
Tsimpida M, Hungerford J, Arora A, Cohen V. Plaque radiotherapy treatment with ruthenium-106 for iris malignant melanoma. Eye. 2011;25:1607–11.
Yousef YA, Finger PT. Lack of radiation maculopathy after palladium-103 plaque radiotherapy for iris melanoma. Int J Radiat Oncol Biol Phys. 2012;83:1107–12.
Konstantinidis L, Roberts D, Errington RD, Kacperek A, Damato B. Whole anterior segment proton beam radiotherapy for diffuse iris melanoma. Br J Ophthalmol. 2013;97:471–4.
Willerding GD, Cordini D, Hackl C, Karle B, Lakotka N, Foerster MH, et al. Proton beam radiotherapy of diffuse iris melanoma in 54 patients. Br J Ophthalmol. 2015;99:812.
Thomson RM, Furutani KM, Pulido JS, Stafford SL, Rogers DW. Modified COMS plaques for 125I and 103Pd iris melanoma brachytherapy. Int J Radiat Oncol Biol Phys. 2010;78:1261–9.
Amin MB, Edge SB. AJCC cancer staging manual. Springer; 2017.
Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31:633–74.
Popovic M, Ahmed IIK, DiGiovanni J, Shields CL. Radiotherapeutic and surgical management of iris melanoma: a review. Surv Ophthalmol. 2017;62:302–11.
Finger PT. Plaque radiation therapy for malignant melanoma of the iris and ciliary body. Am J Ophthalmol. 2001;132:328–35.
Shields CL, Naseripour M, Shields JA, Freire J, Cater J. Custom-designed plaque radiotherapy for nonresectable iris melanoma in 38 patients: tumor control and ocular complications. Am J Ophthalmol. 2003;135:648–56.
Chaugule SS, Finger PT. Regression patterns of iris melanoma after palladium-103 ((103)Pd) plaque brachytherapy. Ophthalmology 2017;124:1023–30.
Agraval U, Sobti M, Russell HC, Lockington D, Ritchie D, Cauchi P, et al. Use of ruthenium-106 brachytherapy for iris melanoma: the Scottish experience. Br J Ophthalmol. 2018;102:74–8.
Liu W, Kim JM, Young BK, Nath R, Chen Z, Decker RH, et al. Novel eye plaque designs for brachytherapy of iris and ciliary body melanoma and the first clinical application. Ocul Oncol Pathol. 2019;5:220–8.
Scanderbeg DJ, Vasudev D, Rice RK, Goldbaum M, Mundt AJ. A modified COMS plaque for iris melanoma. J Contemp Brachytherapy. 2011;3:131–3.
Chiu-Tsao S-T, Astrahan MA, Finger PT, Followill DS, Meigooni AS, Melhus CS, et al. Dosimetry of 125I and 103Pd COMS eye plaques for intraocular tumors: report of Task Group 129 by the AAPM and ABS. Med Phys. 2012;39:6161–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, T.T., Pulido, J.S., Deufel, C.L. et al. Clinical outcomes of Modified Collaborative Ocular Melanoma Study IRIS plaques for treatment of iris, iridociliary, and ciliary body melanoma. Eye 35, 2754–2762 (2021). https://doi.org/10.1038/s41433-020-01295-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01295-y