Abstract
Background/objectives
To prospectively evaluate changes in peripapillary retinal nerve fibre layer (pRNFL), in all macular layers and in choroidal thickness (CT) in a cohort of systemic lupus erythematosus (SLE) patients without ophthalmologic manifestations. To associate those changes with ophthalmic characteristics, disease activity state, medication and systemic comorbidities.
Subjects/methods
Prospective cohort study of 68 previously diagnosed SLE patients. In two study visits (V1 and V2) at least 12 months apart, patients underwent a complete ophthalmologic examination including spectral domain-optical coherence tomography (SD-OCT) and an autoimmune disease specialist assessment. Automatic retinal segmentation was performed. pRNFL was determined globally and in the six peripapillary sectors and each macular layer thickness was determined in the nine early treatment diabetic retinopathy study (ETDRS) subfields. CT was manually measured at 13 locations in the posterior pole. Only one eye per patient was randomly selected for inclusion. Generalised linear mixed effects models were employed.
Results
Sixty-five patients completed the study. The median follow-up time was twelve months. At V2, pRNFL was significantly thinner globally (p = 0.006) and in the temporal inferior sector (p = 0.017). Patients under chronic medication with anticoagulants or antihypertensives had significantly thinner pRNFL in some locations. No significant changes were observed in macular layers or choroidal thickness between study visits.
Conclusions
SLE patients presented early SD-OCT signs of neurodegeneration, evidenced by a progressive reduction in pRNFL thickness. Regardless of study visit, baseline chronic medication with anticoagulants or antihypertensives was associated with lower pRNFL thickness, accounting for a deleterious effect of cardiovascular risk factors.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect any organ system. Nervous system involvement is common, with a prevalence ranging from 12 to 95% among SLE patients [1]. It is responsible for a wide variety of nonspecific and heterogeneous neuropsychiatric manifestations brought together by the American College of Rheumatology under the designation of neuropsychiatric lupus (NP-SLE). Nineteen neuropsychiatric syndromes have been defined, divided into twelve central nervous system (CNS) and seven peripheral nervous system syndromes [2]. These syndromes range from hazardous and disabling conditions such as stroke, aseptic meningitis and seizures to more subtle manifestations like headache, mood disorders and cognitive dysfunction [2]. Overall, NP-SLE is associated with a significant decrease in patient-reported quality of life and with a significant increase in mortality [3, 4]. However, the frequency of some sort of neurologic involvement is unexpectedly high, even in patients without overt neuropsychiatric manifestations. Compared to age-matched controls, non-NP-SLE patients have higher rates of structural CNS abnormalities detected by magnetic resonance imaging (MRI) and present abnormal patterns of CNS activation in response to cognitive tasks in studies with functional MRI or positron emission tomography scan [5,6,7,8]. These patients also perform worse in a variety of cognitive tests and a significant increase in the risk of dementia among SLE patients in population-based large scale studies has been identified [9, 10]. Taken together, these facts support the idea of a chronic relentless neurodegenerative process beginning early in the course of the disease.
The retina is a neuronal tissue with the same embryologic origin as the brain, allowing for the study of CNS alterations. Spectral domain-optical coherence tomography (SD-OCT) is a non-invasive, reliable and widely available examination that provides high resolution cross-sectional images of the retina, choroid and optic nerve. It also enables the measurement of the thickness of each retinal layer and the choroid with high reproducibility and repeatability [11]. Peripapillary retinal nerve fibre layer (pRNFL) thinning detected by SD-OCT, particularly at the temporal sectors, is already a well-established biomarker of neurodegeneration in several disorders, such as Alzheimer’s disease [12], Parkinson’s disease [13] or multiple sclerosis [14]. A few studies evaluated pRNFL in SLE patients and reported a reduction in this structure compared to healthy controls [15,16,17]. A positive correlation between pRNFL thickness in the temporal superior and temporal inferior sectors and the cognitive tests scores has also been reported [16]. A reduction in macular photoreceptors layer has also been reported as an early sign of neurodegeneration in SLE patients, as well as in other disorders like diabetes mellitus and metabolic syndrome [15, 18, 19]. According to the most accepted definition of neurodegeneration, the term is applicable to any pathological condition in which there is primary loss of structure and/or function of neurons, having a chronic relentless course and an essentially unknown cause [20]. As stated, several studies with functional and structural MRI, as well as several studies involving cognitive tests, report a higher rate of abnormal results compared to age-matched controls. Very few studies, however, have demonstrated its chronic and progressive course. Longitudinal cognition studies have given inconsistent results, but several methodological issues limit the utility of these findings [21,22,23]. On the other hand, a prospective study using diffusion tensor imaging MRI revealed progressive microstructural changes in the white and grey matter of SLE patients without NP-SLE over the course of 18 months. These changes occurred without associated progressive cognitive decline or evolving macrostructural changes on MRI [23]. However, to the best of our knowledge, this progressive course has never been addressed in longitudinal studies with SD-OCT in SLE patients.
The objectives of this study were to prospectively evaluate retinal and choroidal changes in a cohort of SLE patients without overt ophthalmic involvement over two follow-up visits, at least 1 year apart, and to relate those changes with existing reports on neurodegenerative changes in SLE patients. We also aimed to study the association between retinal and choroidal structural changes and demographic, ocular and systemic factors, namely age, gender, spherical equivalent, best corrected visual acuity (BCVA), axial length, intraocular pressure (IOP), mean arterial pressure (MAP), body mass index (BMI), systemic medication, disease duration, systemic disease activity index, hydroxychloroquine intake and cumulative dosage, NP-SLE, lupus nephritis, anti-phospholipid syndrome and Sjogren syndrome.
Methods
Patients
This was a prospective cohort study performed at the Ophthalmology Department and at the Autoimmune Disease Units of Central Lisbon Hospital University Centre, between August 2017 and August 2019. Consecutive SLE patients sent to the Ophthalmology Department were screened for inclusion/exclusion criteria. All patients fulfilled the 1997 revised American College of Rheumatology criteria for the diagnosis of SLE [24]. Patients aged between 18 and 80 years old, without signs or antecedents of lupus retinopathy, choroidopathy or optic neuropathy were enroled in the study.
The exclusion criteria were a spherical equivalent >5 dioptres, axial length >25 mm or keratic astigmatism >3 dioptres, diabetes mellitus, pregnancy, signs or previous history of optic neuropathy, retinopathy or choroidopathy (namely lupus-related, age-related macular degeneration, vascular occlusion, macular dystrophy, hydroxychloroquine (HCQ) retinopathy, glaucoma, ocular hypertension or neurodegenerative diseases like Alzheimer’s or Parkinson’s disease), ocular tumour, previous episodes of intraocular inflammation, history of intraocular or refractive surgery and substantial media opacities that compromised fundus imaging.
Due to the exploratory nature of the present study, no previous references about changes of the outcome variables under study after 1 year of follow-up were found. Accordingly, a medium effect size of 0.5 was considered and a sample size of at least 37 patients was obtained, for a level of significance α = 0.05, a power of 0.8, and a percentage of 10% of patients lost to follow-up [25].
This study was approved by our Institutional Ethics Committee. Written informed consent was obtained from all participants and the principles of the Declaration of Helsinki were followed.
Study procedures
Each patient attended two study visits: the inclusion visit (V1) and the follow-up visit (V2). At each time a complete ophthalmic examination, including the clinical history, BCVA testing, slit-lamp biomicroscopy, dilated fundus examination, Goldmann applanation tonometry and SD-OCT (Spectralis® Heidelberg, software version 6.0 Heidelberg, Germany) was performed. The blood pressure was measured before performing SD-OCT. All patients currently or previously medicated with hydroxychloroquine (HCQ) or chloroquine also performed fundus autofluorescence imaging and 10-2 macular automated threshold visual field testing (using Octopus 900®, Haag Streit AG, Koeniz, Switzerland), to exclude retinal toxicity, in accordance with the American Academy of Ophthalmology guidelines [26]. At visit V1, optical biometry (using Lenstar LS 900®, Haag Streit AG, Koeniz, Switzerland) was also performed in order to access axial length. One eye per patient was randomly selected for the study.
All patients were also examined by an autoimmune disease specialist within 1 month of the date of each study visit. These appointments included a complete physical examination and laboratory tests required to access systemic/organ involvement and disease activity (SLEDAI score) [27].
Spectral-domain optical coherence tomography imaging and layer segmentation
Tomographic scans were obtained with Spectralis® SD-OCT in all patients, after pupillary dilation. All exams were performed at the same time in both visits, between 2 and 4 p.m. by an ophthalmologist (JTF) and were assessed by another ophthalmologist (ADS), both masked to the patients’ diagnosis. The study only included good quality scans, chosen on the basis of a signal strength better than 20 (40 = maximum), with well-focused images and continuous scan patterns without artefacts or blank areas.
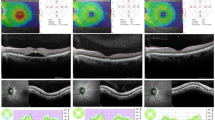
A 12° circular scan centred on the optic nerve head (corresponding to a retinal diameter of ~3.46 mm) was obtained in all patients. pRNFL thickness measurements were determined globally (G) and for the six peripapillary sectors (TS—temporal superior, T—temporal, TI—temporal inferior, NI—nasal inferior, N—nasal, and NS—nasal superior) by the instrument’s built-in software (ʻRNFL Single Exam Report OU with FoDi™’), which automatically segments the internal limiting membrane and the posterior border of the retinal nerve fibre layer (Fig. 1a).
a Peripapillary retinal nerve fibre layer thickness (μm) obtained by ʻRNFL Single Exam Report OU with FoDi™’. b Representative macular thickness map, using ETDRS protocol and macular segmentation using Spectralis automatic segmentation software. c Choroidal thickness measurements (µm) at 13 locations: subfoveally and at 500 μm intervals from the fovea to 1500 μm temporal, 1500 μm nasal, 1500 μm superior and 1500 μm inferior.
Macular scans were also taken using fast macular thickness OCT protocol. Images were then analysed using Spectralis automatic segmentation software in order to calculate individual retinal layer thickness values, namely overall retinal thickness (RT), RNFL, ganglion cell layer (GCL), inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, photoreceptor layer and retinal pigment epithelium. The thickness of each layer was calculated in the nine sectors of the Early Treatment Diabetic Retinopathy Study (ETDRS) plot [28]. This plot, which is automatically projected onto the retina by the Spectralis OCT software, consists of three concentric rings with 1-, 3- and 6-mm diameter, centred on the fovea. The two outer ETDRS rings are crossed by two intersecting lines dividing them into quadrants, generating nine sectors: C, S3, T3, I3, N3, S6, T6, I6 and N6 (Fig. 1b).
Choroidal imaging was also performed using fast macular thickness OCT protocol in the enhanced depth imaging (EDI) mode, according to a previously described method [29]. Choroidal thickness (CT) was measured manually, subfovealy and at 500 μm intervals from the fovea to 1500 μm temporal, 1500 μm nasal, 1500 μm superior and 1500 μm inferior (13 locations) (Fig. 1c).
Image quality, centration and segmentation were verified by two ophthalmologists masked to the patients’ diagnosis and corrected if necessary.
Statistical analysis
Categorical data were expressed as frequencies (percentages), and continuous variables as mean (standard deviation [SD]) or median and interquartile range (25th percentile–75th percentile), as appropriate. Generalised linear mixed effects regression models were used to control for confounding and to identify the variables that explained the variability of thickness of pRNFL, all macular layers and choroid in lupus patients. The effect of age, gender, spherical equivalent, BCVA, axial length, IOP, MAP, BMI, systemic medication, disease duration, systemic disease activity index, hydroxychloroquine intake and cumulative dosage, NP-SLE, lupus nephritis, anti-phospholipid syndrome and Sjogren syndrome was considered in this analysis. In the univariable regression analysis, all the variables that attained a p value ≤ 0.25 were considered for the multivariable models. The normality assumption of the residuals was verified using a Kolmogorov-Smirnov goodness-of-fit test. To study progressive CT decrease from the centre to the periphery, Friedman test was applied. A level of significance α = 0.05 was considered. To cope with the multiple testing problem inherent to this data analysis, Bonferroni correction was used. Data were analysed using STATA 15.0 (StataCorp. 2015. Stata Statistical Software: Release 15. StataCorp LP, College Station, TX).
Results
Eighty-seven SLE patients were initially called for inclusion in the study. Of these, nineteen were excluded based on the exclusion criteria. Therefore, a total of sixty-eight patients were enroled in this study and examined at the inclusion visit (V1). These patients were then called upon for a follow-up visit (V2) after a median time of 12 (interquartile range: 12–17) months. Of the sixty-eight patients initially enroled in the study, only three were lost to follow-up. Overall sixty-five patients completed the study, which corresponds to an attrition rate of 4.6%. None of the patients developed posterior segment manifestations at the end of the follow-up period, namely HCQ retinopathy, lupus choroidopathy or retinopathy or optic neuropathy.
Demographic and clinical characteristics for both study visits (V1 and V2), except for SD-OCT data, are expressed in Table 1. The median duration of SLE diagnosis at V1 was 11.0 (interquartile range: 6.25–19.0) years, with a median HCQ treatment time of 5.3 (interquartile range: 1.81–11.83) years. Of note, these patients were clinically stable, with low disease activity (median SLEDAI: 2) at the time of both visits. While the cumulative dose of HCQ increased from V1 to V2, as expected, the mean daily dose slightly reduced. The mean daily dose of systemic corticosteroids also reduced at the end of the follow-up. Remarkably, all other clinical variables remained relatively stable.
Peripapillary retinal nerve fibre layer thickness
At visit 2, there was a reduction in pRNFL thickness in all sectors, compared to visit 1 (Table 2). This reduction was statistically significant globally (p = 0.006) and in the TI sector (p = 0.017). There was also a weak evidence of a reduction in the NI sector (p = 0.053).
The results of the univariable regression analysis are presented in Supplementary Tables S1–S7. The results of the multivariable regression analysis (Table 3), after adjusting for the effects of gender, age, BCVA, IOP, spherical equivalent, axial length, BMI, MAP, systemic medication, disease duration, disease activity and systemic comorbidities, revealed a statistically significant reduction in pRNFL globally (p = 0.006) and in the TI (p = 0.018) sector. In the NI sector, a weak evidence for decreased thickness in comparison to V1 was observed (p = 0.052). However, after applying Bonferroni adjustment for multiple testing, statistical significance was only observed globally. Age was negatively associated with pRNFL thickness globally and in the TS, TI and NI sectors, with a mean reduction of 0.34–0.56 µm in pRNFL thickness for each additional year of life. After Bonferroni corrections, statistical significance was maintained globally and in the TI sector. Regardless of the study visit, axial length was also negatively associated with pRNFL thickness globally and in the TS, NI and NS sectors, with a mean reduction of 3.65–10.68 µm for each mm increase in axial length. After Bonferroni corrections, statistical significance was observed globally and in the NI and NS sectors. There was also a negative association between baseline chronic medication with anticoagulant and pRNFL thickness, globally and in the TI and NI sectors (mean pRNFL thickness was 6.90–18.21 µm lower in patients taking this medication, independently of the study visit). After Bonferroni adjustment, this association maintained statistical significance in the TI and NI sectors. Finally, a significant association was observed between pRNFL thickness and baseline chronic medication with calcium channel blocker in the NS sector (mean pRNFL thickness was 22.38 µm lower in patients taking this class of drugs, independently of the study visit). This significant association was maintained after Bonferroni adjustment.
Retinal layers thickness
The results of multiple regression analysis for total RT and for all macular layers revealed no significant differences at visit 2 compared to visit 1, after considering the effects of all the other variables. The graphs comparing overall RT and the thickness of all layers in all ETDRS areas between the two study visits are depicted in Fig. 2.
Choroidal thickness
There were no statistically significant differences in CT from visit 1 to visit 2 (Supplementary Table S8). In both visits, SLE patents maintained the same pattern of CT topographic distribution throughout the posterior pole. Along the vertical meridian, the normal progressive decrease of CT from the centre to the periphery was not observed (p > 0.05). Additionally, mean CT was higher in the inferior quadrants compared to the superior quadrants. Regarding the horizontal section, the choroid was thicker temporally and the normal progressive decrease in CT from the centre to the periphery was preserved in both visits (p < 0.001).
Discussion
This prospective study, which enroled sixty-five SLE patients over more than a year, was the first longitudinal study evaluating retinal and choroidal changes in SLE patients with SD-OCT. A significant reduction in pRNFL globally and in the TI sector was documented. Regardless of the study visit, baseline chronic medication with anticoagulants and anti-hypertensive drugs was associated with lower pRNFL thickness in several sectors.
This was the first study to document a progressive decline in pRNFL thickness throughout the follow-up period. pRNFL decreased globally and in the TI sector, after adjusting for the effects of age and all other variables that attained a p value < 0.25 in a univariate model. After applying Bonferroni adjustment for multiple testing, statistical significance was maintained globally. In the remaining peripapillary sectors, pRNFL also reduced, however without statistical significance. As expected, age and axial length presented a negative association with pRNFL in several sectors. The multivariable analysis also revealed a significant negative association between pRNFL thickness and baseline chronic medication with anticoagulants in the TI and NI sectors. Baseline chronic medication with calcium channel blocker also presented a negative association with pRNFL thickness in the NS sector. These results, obtained regardless of the study visit, suggest a deleterious effect of cardiovascular risk factors in neuronal degeneration. In fact, these results are in accordance with previous studies linking metabolic syndrome not only to cardiovascular disease but also to ocular and neurological involvement [30, 31].
Cross-sectional and case control studies on RT in SLE have yielded conflicting results [15,16,17, 32]. A recent study prospectively evaluated total RT and GCL thickness in SLE patients taking HCQ for five years and found a statistically significant reduction in both, without qualitative changes in the external retinal layers. However, given the fact that a multivariable analysis was not performed, the effect of other contributing factors like aging was not accounted for [33]. In our study we did not find consistent differences in any retinal layers or in total RT after adjusting for the effects of age, disease activity, systemic involvement and all other variables; however, our follow-up period was shorter.
CT analysis revealed no significant changes over the follow-up period. These patients presented, though, an abnormal CT distribution on the posterior pole that was maintained throughout the follow-up period. The normal progressive decrease in CT from the centre to the periphery was not observed in the vertical meridian and the mean CT was higher in the inferior quadrants compared to the superior quadrants, as opposed to what happens in healthy individuals. On the other hand, along the horizontal foveal section, the relationships were preserved as the choroid was thicker temporally than nasally, with a normal progressive decrease in thickness from the centre to the periphery [34, 35]. These findings may reflect subclinical signs of choroidopathy and are in accordance with those of previous studies using indocyanine green angiography [36].
The concept of SLE as a neurodegenerative disorder is still a debatable issue. Besides the above-mentioned structural and functional changes in the CNS of SLE patients, other arguments support the existence of premature neural tissue damage, even in asymptomatic patients. Previous studies revealed an increase in cerebrospinal fluid levels of molecules indicating neuronal and astrocytic damage, namely neurofilament (a neuronal degradation product), Tau (an axonal degeneration product) and astroglial fibrillary acidic protein, compared to healthy controls [37, 38]. Additionally, histological studies demonstrate increased neuronal apoptosis in mice models of SLE [39, 40]. The reduction in pRNFL observed in our study might thus be a subtle indirect sign of progressive axonal degeneration.
This study has some limitations. The first limitation is the follow-up time. Twelve months may be a short period in what concerns neurodegenerative changes. Although a significant reduction in pRNFL has been observed in this period, a longer follow-up might have enabled the observation of macular layers changes. Despite this fact, this has been the first prospective study evaluating this outcome with SD-OCT and has a similar follow-up time as a previous prospective MRI study that unveiled progressive CNS microstructural changes in SLE patients [23]. Second, automatic retinal segmentation was employed to measure retinal layers thickness. Although this methodology is susceptible to segmentation errors, it has already proven high repeatability and reproducibility [11, 41]. Moreover, detailed post-segmentation inspection and refinement by a trained ophthalmologist was performed whenever segmentation was considered inaccurate. Another limitation concerns CT measurements, which were obtained manually. However, this technique has demonstrated high intraobserver and interobserver reproducibility [42]. Finally, our assessment of disease duration was based on the time of the diagnosis. This may, in some cases, have underestimated the real duration of the disease.
In summary, SLE patients present a progressive thinning of pRNFL over the follow-up of 1 year. These microstructural changes might relate to chronic low-grade inflammation, leading to neuronal apoptosis, which are the hallmarks of neurodegeneration. While these interesting results may shed some light into the pathophysiology of progressive nervous tissue changes associated to SLE, one must keep in mind that the magnitude of pRNFL thickness reduction is rather small. Additionally, the effect of confounding factors, like natural ageing and axial length, makes it unsuitable for diagnostic purposes. It might, at most, become a useful tool to monitor the rate of neural tissue loss in SLE patients. Therefore, future studies with longer follow-up periods are needed to confirm the progressive nature of retinal structural changes and to relate them with structural and functional changes in CNS imaging. Additionally, they should include larger cohorts, preferably sampled by risk factors and comorbidities. These would enable more solid conclusions particularly on a potential deleterious effect of cardiovascular risk factors, as suggested by the results of this study.
Summary
What was known before
-
Asymptomatic patients with SLE present higher rates of structural and functional CNS changes compared to age-matched controls.
-
These findings support the idea of a progressive neurodegenerative process associated to this autoimmune disease.
-
Optical coherence tomography has already proven its role in detecting subtle retinal neurodegenerative changes in some neurodegenerative disorders.
What this study adds
-
This study, which included the first prospective analysis of retinal and CT changes in SLE patients over the course of 1 year, revealed a significant reduction in the pRNFL.
-
Chronic medication with anticoagulants and calcium channel blockers were significantly associated with greater pRNFL thinning in some sectors, suggesting a deleterious effect of cardiovascular risk factors.
References
Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28.
Liang MH, Corzillius M, Bae SC, Lew RA, Fortin PR, Gordon C, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608.
Mak A, Cheung MWL, Chiew HJ, Liu Y, Roger Chun-man HO. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41:830–9.
Hanly JG, Urowitz MB, Jackson D, Bae SC, Gordon C, Wallace DJ, et al. SF-36 summary and subscale scores are reliable outcomes of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:961–7.
Sarbu N, Toledano P, Calvo A, Roura E, Sarbu MI, Espinosa G, et al. Advanced MRI techniques: biomarkers in neuropsychiatric lupus. Lupus. 2017;26:510–6. https://doi.org/10.1177/0961203316674820.
Sarbu N, Alobeidi F, Toledano P, Espinosa G, Giles I, Rahman A, et al. Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmun Rev. 2015;14:153–9.
Zhu CM, Ma Y, Xie L, Huang JZ, Sun ZB, Duan SX, et al. Spatial working memory impairment in patients with non-neuropsychiatric systemic lupus erythematosus: a blood-oxygen-level dependent functional magnetic resonance imaging study. J Rheumatol. 2017;44:201–8.
Ramage AE, Fox PT, Brey RL, Narayana S, Cykowski MD, Naqibuddin M, et al. Neuroimaging evidence of white matter inflammation in newly diagnosed systemic lupus erythematosus. Arthritis Rheum. 2011;63:3048–57.
Monastero R, Bettini P, Del Zotto E, Cottini E, Tincani A, Balestrieri G, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184:33–9.
Gendelman O, Tiosano S, Shoenfeld Y, Comaneshter D, Amital H, Cohen AD, et al. High proportions of dementia among SLE patients: a big data analysis. Int J Geriatr Psychiatry. 2018;33:531–6.
Ctori I, Huntjens B. Repeatability of foveal measurements using spectralis optical coherence tomography segmentation software. PLoS ONE. 2015;10:e0129005.
Cunha JP, Proença R, Dias-Santos A, Almeida R, Águas H, Alves M, et al. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch Clin Exp Ophthalmol. 2017;255:1827–35.
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vis Res. 2004;44:2793–7.
Toledo J, Sepulcre J, Salinas-Alaman A, García-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906–12.
Dias-Santos A, Tavares Ferreira J, Pinheiro S, Cunha JP, Alves M, Papoila AL, et al. Neurodegeneration in systemic lupus erythematosus: Layer by layer retinal study using optical coherence tomography. Int J Retina Vitreous. 2020;6:15. https://doi.org/10.1186/s40942-020-00219-y. Accessed 1 May 2020.
Liu GY, Utset TO, Bernard JT. Retinal nerve fiber layer and macular thinning in systemic lupus erythematosus: an optical coherence tomography study comparing SLE and neuropsychiatric SLE. Lupus. 2015;24:1169–76.
Shulman S, Shorer R, Wollman J, Dotan G, Paran D. Retinal nerve fiber layer thickness and neuropsychiatric manifestations in systemic lupus erythematosus. Lupus. 2017;26:1420–5.
Tavares Ferreira J, Alves M, Dias-Santos A, Costa L, Santos BO, Cunha JP, et al. Retinal neurodegeneration in diabetic patients without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2016;57:6455–60.
Karaca C, Karaca Z. Beyond hyperglycemia, evidence for retinal neurodegeneration in metabolic syndrome. Investig Ophthalmol Vis Sci. 2018;59:1360–7.
Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Investig. 2003;111:3–10.
Carlomagno S, Migliaresi S, Ambrosone L, Sannino M, Sanges G, Di, et al. Cognitive impairment in systemic lupus erythematosus: a follow-up study. J Neurol. 2000;247:273–9.
Hanly JG, Fisk JD, Sherwood G, Eastwood B. Clinical course of cognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1994;21:1825–31.
Kozora E, Filley CM, Erkan D, Uluğ AM, Vo A, Ramon G, et al. Longitudinal evaluation of diffusion tensor imaging and cognition in systemic lupus erythematosus. Lupus. 2018;27:1810–8.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Ryan TP. Sample size determination and power. New Jersey: John Wiley & Sons; 2013.
Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF, Lum F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386–94.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Austin A, et al. Derivation of the sledai. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. http://www.ncbi.nlm.nih.gov/pubmed/1599520.
Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103:1796–806.
Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Demir S, Artim-Esen B, Şahinkaya Y, Pehlivan, Alpay-Kanltez N, Omma A, et al. Metabolic syndrome is not only a risk factor for cardiovascular diseases in systemic lupus erythematosus but is also associated with cumulative organ damage: a cross-sectional analysis of 311 patients. Lupus. 2016;25:177–84.
Mok CC, Tse SM, Chan KL, Ho LY. Effect of the metabolic syndrome on organ damage and mortality in patients with systemic lupus erythematosus: a longitudinal analysis. Clin Exp Rheumatol. 2018;36:389–95.
Conigliaro P, Triggianese P, Draghessi G, Canofari C, Aloe G, Chimenti MS, et al. Evidence for the detection of subclinical retinal involvement in systemic lupus erythematosus and sjögren syndrome: a potential association with therapies. Int Arch Allergy Immunol. 2018;177:45–56.
Martín-Iglesias D, Artaraz J, Fonollosa A, Ugarte A, Arteagabeitia A, Ruiz-Irastorza G. Evolution of retinal changes measured by optical coherence tomography in the assessment of hydroxychloroquine ocular safety in patients with systemic lupus erythematosus. Lupus. 2019;28:555–9.
Esmaeelpour M, Považay B, Hermann B, Hofer B, Kajic V, Kapoor K, et al. Three-dimensional 1060-nm OCT: Choroidal thickness maps in normal subjects and improved posterior segment visualization in cataract patients. Investig Ophthalmol Vis Sci. 2010;51:5260–6.
Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Investig Ophthalmol Vis Sci. 2010;51:2173–6.
Baglio V, Gharbiya M, Balacco-Gabrieli C, Mascaro T, Gangemi C, di Franco M, et al. Choroidopathy in patients with systemic lupus erythematosus with or without nephropathy. J Nephrol. 2011;24:522–9.
Trysberg E, Höglund K, Svenungsson E, Blennow K, Tarkowski A. Decreased levels of soluble amyloid beta-protein precursor and beta-amyloid protein in cerebrospinal fluid of patients with systemic lupus erythematosus. Arthritis Res Ther. 2004;6:R129–36.
Trysberg E, Nylen K, Rosengren LE, Tarkowski A. Neuronal and astrocytic damage in systemic lupus erythematosus patients with central nervous system involvement. Arthritis Rheum. 2003;48:2881–7.
Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun. 2015;60:40–50.
Lu XY, Chen XX, Huang LD, Zhu CQ, Gu YY, Ye S. Anti-alpha-internexin autoantibody from neuropsychiatric lupus induce cognitive damage via inhibiting axonal elongation and promote neuron apoptosis. PLoS One. 2010;5:e11124.
Çetinkaya E, Duman R, Duman R, Sabaner MC. Repeatability and reproducibility of automatic segmentation of retinal layers in healthy subjects using Spectralis optical coherence tomography. Arq Bras Oftalmol. 2017;80:378–81.
Shao L, Xu L, Chen CX, Yang LH, Du KF, Wang S, et al. Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2013;54:230–3.
Funding
A grant for this study was given by José de Mello Saúde—Hospital CUF Descobertas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dias-Santos, A., Tavares Ferreira, J., Pinheiro, S. et al. Retinal and choroidal thickness changes in systemic lupus erythematosus patients: a longitudinal study. Eye 35, 2771–2780 (2021). https://doi.org/10.1038/s41433-020-01292-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01292-1
This article is cited by
-
Choroidal involvement in systemic vasculitis: a systematic review
Journal of Ophthalmic Inflammation and Infection (2022)