Abstract
Background
Increasing demand on hospital services has led to the development of alternative community-based services, often run by optometrists for monitoring ‘stable’ and low-risk glaucoma patients.
Methods
An online Delphi exercise was undertaken to derive a consensus definition of ‘stable glaucoma’ amongst optometrists with a special interest in glaucoma. Participants were asked to score their agreement for various clinical parameters. Results from each round were used to inform subsequent rounds.
Results
31 optometrists participated in the study. 100%, 77%, and 68% completion rates were achieved over three rounds respectively. Consensus was reached for 7 parameters: Stability should be defined over a period of 36–48 months, summary measure Visual Field (VF), and/or Trend Analysis should be used to assess VF stability. Two or more decibel (dB) of change of VF mean deviation (MD) is considered unstable. Intraocular pressure (IOP) should be below a target defined by the patient’s clinician or a fixed-percentage reduction compared to the presenting IOP. No treatment change during the stability assessment period is considered stable. Imaging with Ocular Coherence Topography Retinal Nerve Fibre Layer (OCT RNFL) assessment should be used to define glaucoma stability. Overview by a glaucoma consultant was considered important for glaucoma monitoring schemes.
Conclusion:
This Delphi exercise has generated a consensus definition for glaucoma stability by UK Optometrists with a specialist interest in glaucoma. This consensus definition can be used to inform the selection of suitable patients from hospital services for transfer to monitoring in community-based ‘stable’ optometry run glaucoma clinics.
Similar content being viewed by others
Introduction
Every year, secondary care Ophthalmology services receive over 172,000 referrals for patients with ‘suspect’ glaucoma [1]. In addition to these new referrals, over 30% of ongoing glaucoma reviews within hospital eye services (HES) are for ocular hypertension (OHT) and suspected glaucoma [2]. These figures are set to rise as the Royal College of Ophthalmologists (RCOphth) predicts a 44% increase in glaucoma cases over the next 20 years [3]. This rising demand on finite hospital services highlights the importance of developing and refining alternative community-based services often provided by optometrists for monitoring ‘stable’ and low-risk glaucoma patients [1, 3]. Optometrist involvement in alternative models of glaucoma service provision in the UK is well established; from working within HES to community referral refinement schemes [4,5,6] and stable glaucoma monitoring schemes [7, 8].
The National Institute for Health and Care Excellence (NICE) guidelines for managing glaucoma outline the general principles and investigations for monitoring OHT, glaucoma suspect, and chronic open-angle glaucoma (COAG) patients [9]. NICE estimates that approximately one third (56,320 out of the 169,500) of patients with COAG, suspect COAG, and OHT currently being managed in secondary care could be managed in the community [9].
The question then arises, how do we define ‘stability’ for monitoring purposes and identify low-risk patients that are suitable for transferring into the community. At present, there is no established consensus on the clinical definition of ‘Stable Glaucoma’ available in the literature. This definition is left to the discretion of local service providers leading to inconsistency within and between glaucoma departments and community-based schemes.
A definition of ‘stable glaucoma’ would inform the effective design and commissioning of glaucoma services in the National Health Service (NHS) by identifying patients who can be monitored in the community, contribute to developing standards for these patients to be managed safely and aid in accurately and consistently identifying those who need to be re-referred back to secondary care.
As the majority of community-based glaucoma services are run by optometrists with specialist glaucoma qualifications [10] and have varying degrees of ophthalmologist oversight—the optometric interpretation of ‘stable glaucoma’ is important and relevant in identifying suitable patients for these schemes.
The aim of this study is:
-
1.
To establish a consensus on the definition of ‘stable’ glaucoma amongst optometrists with a special interest in glaucoma.
-
2.
To evaluate which factors are important when discharging ‘stable glaucoma’ patients to community-based schemes.
-
3.
Provide guidance on the parameters of ‘stable glaucoma’ for clinicians involved in community-based services.
Method
To ensure a representative sample, 31 optometrists with a special interest in glaucoma based in Nottingham, Manchester, and Bristol hospital departments, representative for the range of typical glaucoma units across the country were approached to participate in the study. This panel was then consulted [11,12,13] to establish consensus on the definition of stable glaucoma. The University of Nottingham School of Medicine Ethics committee confirmed that this consultative survey did not require ethical approval.
The panel was provided with information about the survey and subsequently participated in a 3 round Delphi exercise via the online survey tool Survey Monkey (https://www.surveymonkey.com/). Each participant was sent a personalised link and asked to indicate their strength of agreement for a series of parameters using a 0–10 scoring scale, where 0 indicated strong disagreement and 10 indicated strong agreement. Each survey round was designed to take around 15 min to complete.
The clinical parameters explored through 67 questions were (Fig. 1) (see Supplementary information appendix):
-
1.
Time Period: After what monitoring period is a patient’s glaucoma considered ‘stable’?
-
2.
Visual Field Methods: Which assessment methods should be used to define ‘stability’?
-
3.
Imaging Methods: Which imaging assessment methods should be used to define optic nerve head ‘stability’?
-
4.
Intra-ocular pressure (IOP): What IOP level indicates ‘stability’?
-
5.
Use of drops: Can the total number of IOP lowering agents drops being used by a patient or a change in number of drops be used to indicate ‘stability’?
-
6.
Consultant Oversight: What sub-speciality clinical expertise should consultants overseeing community monitoring services have?
-
7.
What patient factors need to be considered prior to transferring patients into the community?
Based on the NICE glaucoma guidelines, it was anticipated that respondent’s answers would assume IOP measurement using Goldmann applanation tonometry and VF assessment using automated perimetry. After each round, scores were assessed and descriptive statistics were generated for each parameter. A group median score 8–10 was considered to indicate ‘strong agreement with a median score of 0–2 representing strong disagreement. The use of median scores to summarise group responses in this way is common in Delphi research [13] however, median scores in isolation may disguise a broad range of scores which might be typical of panel disagreement. To counter this and to add rigour to our Delphi process, we combined a median score with an Interquartile Range (IQR) assessment [12,13,14,15]. An IQR score indicates the spread of scores across the range of scoring options; an IQR of 2 indicates that 50% or more of responses are within 1 score of the median, an IQR of 8 indicates that scores are more broadly dispersed. To be confident that agreement about parameters had been reached we defined consensus as a median score indicating strong agreement [8,9,10] or strong disagreement (0–2) in combination with an IQR of 2 points or less (demonstrating a concentration of scoring around the median). In all other circumstances, when there were lower levels of agreement/disagreement (median 3–7) or dispersed scoring (IQR>2), consensus was not reached. Alongside the scoring, participants were also given the opportunity to offer free-text comments which might contextualize or explain their responses and allow expansion of options for consideration.
Those parameters where scoring demonstrated consensus amongst the expert panel were accepted as a characteristic of stable glaucoma. These questions were then closed and not presented in subsequent survey rounds.
Where consensus for a parameter was not achieved, questions were amended (in accordance with previous scoring and any relevant free-text comments) by re-wording in such a way as to support the generation of consensus. For example, the duration of time for monitoring stable glaucoma was increased to support the generation of panel agreement about it. Revised parameters, along with summary scores from previous rounds and any indication of changes to the parameter, were included in the next iteration of the survey for scoring. This process was repeated twice in this amended, 3-round Delphi exercise.
Results
In round 1 there were 31 responses (100%), 24 in round 2 (77%) and 21 in round 3 (68%) [16]. All clinical parameters explored reached consensus. Ten questions achieved consensus in the first round, ten in the second, and fifteen in the third. The results for each clinical parameter are presented in Table 1.
Consensus was achieved that 36–48 months of monitoring is sufficient to consider patients with OHT/primary open-angle glaucoma (POAG) (Round 2 (R2) Median 9 (M9) Interquartile Range 2 (IQR2), R2 M10 IQR1), ‘high’ risk glaucoma (R3 M9 IQR2, R3 M10 IQR0) and ‘low’ risk glaucoma (R3 M10 IQR1, R3 M10 IQR0)—stable.
Consensus was achieved that visual field stability should be assessed by summary measures of Visual Field Index (VFI)/ Mean deviation (MD) progression (R2 M8 IQR2) or trend analysis alone (R2 M9 IQR1). If using a combination of methods, summary measures of VFI/ MD along with Trend analysis (R2 M8 IQR2), or a combination of all three—VFI/ MD, Pointwise progression and Trend analysis (R2 M10 IQR0) can be used together. Consensus was achieved that <2dB and <4dB of change in MD is unstable, no consensus was reached for 0dB and <1dB.
Consensus was achieved that IOP control can be considered stable if IOP is either below a target IOP defined by the patient’s clinician (R1 M8 IQR2) or below a fixed percentage (%) reduction compared to the presenting IOP (R3 M8 IQR2).
Consensus was achieved that no change in treatment during the assessment period is considered stable (R2 M10 IQR1) whilst 3 drop changes is considered unstable (R1 M0 IQR1). No consensus was reached on 2 drop changes or the number of agents required for the optimisation of IOP control being an important factor to consider for stability.
Consensus was achieved that when choosing from a single imaging technique Ocular Coherence Topography Retinal Nerve Fibre Layer (OCT RNFL) assessment (R1 M9 IQR2) should be used to define structural glaucoma stability. In terms of combinations of techniques, Optic disc photos and OCT RNFL assessment can be used together (R2 M8 IQR2).
For all independent community glaucoma monitoring schemes run by optometrists with Glaucoma Higher Qualifications as outlined in the Common Clinical Competency Framework (OCCCF) for Non-medical Ophthalmic Healthcare Professions in Secondary Care: Glaucoma [17], there was strong agreement consensus that they should be overseen by consultants with glaucoma speciality expertise. (R1 M10 IQR2)
For all community scheme models: (1) without consultant overview (R1 M10 IQR1), (2) overseen by general ophthalmologists (R1 M10 IQR1) and (3) overseen by a consultant with glaucoma expertise (R1 M10 IQR0)—there was strong consensus agreement that an assessment of glaucoma stability should be made before patients are transferred to a glaucoma-monitoring scheme.
Only patients with ‘stable’ glaucoma should be transferred to schemes without consultant overview or those overseen by a consultant with glaucoma expertise, no consensus reached for schemes overseen by a general ophthalmologist.
Severity, type of glaucoma and patients age were generally regarded important factors to consider when discharging patients to a community-based monitoring scheme. No consensus was reached for type of glaucoma or patient age being an important factor to consider when discharging to a community scheme with glaucoma consultant oversight.
Discussion
Since its first use in the United States Air-Force to reach a consensus amongst military experts in the RAND project in the 1950’s [18], the Delphi method has become an establish method of consensus development within medicine [19,20,21] and the glaucoma field [22,23,24,25,26,27,28,29,30].
Consensus was reached for 35 questions across all the fundamental clinical parameters explored.
-
(1)
Monitored over 48 months
-
(2)
IOP control below a target defined by the patients’ clinician
-
(3)
Visual field loss monitored by Summary measures of VFI/ MD progression
-
(4)
<2 dB of change in MD
-
(5)
OCT RNFL assessment of Optic nerve head stability
-
(6)
No change to the medication regime
Consensus on the delivery community glaucoma monitoring schemes is that:
-
(1)
They should be overseen by consultants with glaucoma speciality expertise
-
(2)
Assessment of glaucoma stability should be made before patients are transferred to a
-
(3)
glaucoma-monitoring scheme
-
(4)
Only patients with ‘stable’ glaucoma should be transferred to these schemes
-
(5)
Severity, type of glaucoma and patients age should be considered before discharging patients to a community-based monitoring scheme.
This is the first attempt to generate a consensus definition of ‘stable glaucoma’ amongst allied health professionals involved in the delivery of glaucoma care in primary and secondary care settings.
The opinion of optometrists with a specialist interest in glaucoma is essential to the configuration and development of community schemes. These optometrists will be primarily responsible for delivery of these services and thus their views on patient suitability are vital for the effective delivery of community schemes. In addition, as key members of commissioning committees, optometrists play a crucial role in the commissioning of these community schemes.
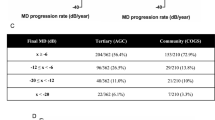
In comparison to a recent study [22] using similar methodology to generate a consensus definition of ‘stable glaucoma’ amongst UK based glaucoma consultants, consensus between optometrists was reached for a greater number of parameters (35 vs 21), in earlier rounds (Fig. 2).
In addition, consensus was reached for the definition of stability across all clinical parameters which was not the case in the consultant exercise. This suggests that there is greater agreement between specially trained and accredited Optometrists working within glaucoma schemes than amongst Glaucoma consultants. The lack of consensus between consultants in comparison to optometrists may be explained by a difference in practice populations exposure. In the current NHS secondary care setting, there are far fewer stable patients as these have largely been transferred to low-risk glaucoma monitoring clinics in hospital and community settings. Consultant practice is largely focused on complex, high-risk patients—this may impact consultant perspective when attempting to define ‘stable glaucoma’.
The corollary of this case mix difference is that optometrists involved in delivery of glaucoma monitoring schemes are primarily exposed to less severe or complex non-progressing patients which may provide them with the confidence to express an opinion on parameters for stability which are more consistent and allow consensus to be achieved.
Despite the difference in the number of parameters reaching consensus, there is considerable overlap between consultant and optometrist agreement for the parameters where consensus was reached and there were no parameters where they disagreed (Table 2).
In the Delphi survey of Glaucoma Consultants; although there was a trend of agreement for longer durations of follow up, no consensus was reached for the monitoring time period required to define stability. There was strong agreement on IOP stability was below a target defined by the patient’s clinician and no consensus on that reduced by a fixed percentage. The number of agents required for IOP optimisation was deemed an important consideration for stability and there was no agreement on a single imaging modality.
It is acknowledged that differences in group composition may lead to a difference in judgements. This sampling issue may reflect degree of knowledge or area of practice—thus combining results from the survey of Glaucoma consultants and that of Glaucoma specialist optometrists would give us the most robust definition of ‘stable glaucoma’.
There have been four published studies comparing the clinical management between consultant glaucoma ophthalmologists and glaucoma specialist optometrists.
Azuara-Blanco et al explored the accuracy of accredited glaucoma optometrists working in the community, in the diagnosis and treatment recommendation for glaucoma in comparison to a hospital-based glaucoma consultant [31], two papers compared the management of glaucoma patients between glaucoma specialist optometrists and ophthalmologists in a hospital setting [32, 33], and one paper assessed the decision making of glaucoma specialist optometrists vs ophthalmologists within a shared care community scheme [34]. All found high levels of agreement between the two professional groups which is consistent with the findings of this study.
Based on the combined findings of both Delphi exercises, we suggest that the following can be used as a practical, working definition of stable glaucoma:
Glaucoma may be defined as ‘stable’ when
-
Monitored over a 48-month period.
-
IOP remains below the target IOP defined by the patients’ clinician, or a fixed % reduction compared to the presenting IOP.
-
There are no medication changes during this period.
-
There is <2dB of change in MD.
Independent community glaucoma monitoring schemes should be overseen by consultants with glaucoma speciality expertise. Prior to discharging patients to a glaucoma-monitoring scheme assessment of glaucoma stability should be made. Only patients with ‘stable’ glaucoma should be transferred to these schemes and severity; type of glaucoma and patients age should be considered before discharging patients to a community-based monitoring scheme.
Our aim was to generate a consensus agreement for defining stable glaucoma to develop a criterion for patient discharge from secondary care to community monitoring schemes and to determine the ideal level of oversight required for these schemes.
This consensus definition of ‘stable glaucoma’ will allow clear Identification of patients currently in secondary care services that are suitable for community monitoring, help to set monitoring parameters for patients within community schemes and to identify those who need to be re-referred back to secondary care resulting in increased consistency and transparency within glaucoma service provision.
Limitations
In this survey, we explored the definition of stable glaucoma using the current standard clinical parameters recommended by NICE to evaluate and monitor glaucoma. This provides a pragmatic approach, familiar and available to all respondents. However, in the future it is possible that additional clinical parameters may be measured routinely and become part of the standard monitoring process. In the case of pachymetry and hysteresis, both are incorporated into the measurement of IOP undertaken by the ocular response analyser and this may become standard practise for assessment of IOP in future. Such developments would be important to include in future studies.
Although the optometrists recruited for this panel predominantly work in a hospital setting, arguably they might be considered to be also representative of those who work in the community, as there is an overlap in their training and accreditation and in at least a proportion of the glaucoma case mix they are likely to have encountered. Published evidence shows that the selection of the participants has little impact on the group decision as long as the selection reflects the range of experience and characteristics of the population from which the participants are selected [35]. Having less than six participants has low reliability and with large groups (above 12) the increase in reliability needs to be balanced with diminishing return rates [11]. Thus, reliable outcomes can be obtained with a relatively small Delphi panel size with a response rate of over 70% [16].
Conclusion
We believe this study has achieved a practical consensus definition of ‘stable’ glaucoma for evaluation of transfer of patients to primary care glaucoma monitoring schemes and a consensus that all such schemes should have glaucoma consultant oversight. This consensus will aid planning and allow consistent modelling of future primary care glaucoma monitoring schemes.
Summary
What was known before
-
The rising demand on finite hospital Ophthalmology services highlights the importance of developing and refining alternative community-based services for monitoring stable and low-risk glaucoma patients. The question then arises, how do we define stability for monitoring purposes and identify low-risk patients that are suitable for transferring into the community. At present, there is no established consensus on the clinical definition of Stable Glaucoma available in the literature. This definition is left to the discretion of local service providers leading to inconsistency within and between glaucoma departments and community-based schemes. A definition of stable glaucoma would inform the effective design and commissioning of glaucoma services in the National Health Service (NHS) by identifying patients who can be monitored in the community, contribute to developing standards for these patients to be managed safely and aid in accurately and consistently identifying those who need to be re-referred back to secondary care.
What this study adds
-
Glaucoma may be defined as stable when: monitored over a 48-month period, IOP remains below the target IOP defined by the patients clinician, or a fixed % reduction compared to the presenting IOP, there are no medication changes during this period, there is <2 dB of change in MD Independent community glaucoma monitoring schemes should be overseen by consultants with glaucoma speciality expertise. Prior to discharging patients to a glaucoma-monitoring scheme assessment of glaucoma stability should be made. Only patients with stable glaucoma should be transferred to these schemes and severity; type of glaucoma and patients age should be considered before discharging patients to a community-based monitoring scheme. We believe this study has achieved a practical consensus definition of stable glaucoma for evaluation of transfer of patients to primary care glaucoma monitoring schemes and a consensus that all such schemes should have glaucoma consultant oversight. This consensus will aid planning and allow consistent modelling of future primary care glaucoma monitoring schemes.
References
Ophthalmologists. The college of Optometrists and The Royal College of. Commissioning better health care: Glaucoma. 2013. http://www.locsu.co.uk/uploads/enhanced_pathways_2013/joint_colleges_glaucoma_guidance.pdf.
Ophthalmologists. The Royal College of. Commissioning guide: Glaucoma. 2016. https://www.rcophth.ac.uk/wp-content/uploads/2016/06/Glaucoma-Commissioning-Guide-Recommendations-June-2016-Final.pdf.
Ophthalmologists. The Royal College of. The Way Forward: Glaucoma. 2018. https://www.rcophth.ac.uk/wp-content/uploads/2015/10/RCOphth-The-Way-Forward-Glaucoma-300117.pdf.
Devarajan N, Williams GS, Hopes M, O’Sullivan D, Jones D. The carmarthenshire glaucoma referral refinement scheme, a safe and efficient screening service. Eye. 2011;25:43–9.
Bourne RR, French KA, Chang L, Borman AD, Hingorani M, Newsom WD. Can a community optometrist-based referral refinement scheme reduce false-positive glaucoma hospital referrals without compromising quality of care? The community and hospital allied network glaucoma evaluation scheme (CHANGES). Eye. 2010;24:881–7.
Henson DB, Spencer AF, Harper R, Cadman EJSL. Community refinement of glaucoma referrals. Eye. 2002;16:1–6.
Gray SF, Spry PG, Brookes ST, Peters TJ, Spencer IC, Baker IA, et al. The Bristol shared care glaucoma study: outcome at follow up at 2 years. Br J Ophthalmol. 2000;84:456–63.
Syam P, Rughani K, Vardy SJ, Rimmer T, Fitt A, Husain T, et al. The Peterborough scheme for community specialist optometrists in glaucoma: a feasibility study. Eye. 2010;24:1156–64.
NICE. Guideline. NICE guidelines. 2017. https://www.nice.org.uk/guidance/ng81/evidence/full-guideline-pdf-4660991389.
Vernon Stephen, Adair A. Shared care in glaucoma: a national study of secondary care lead schemes in England. Eye. 2009;24:265–9.
Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al. Consensus development methods, and their use in clinical guideline development: a review. Health Technol Assess. 1998;2:1–88.
Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;64:401–9.
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review. Plos ONE 2011;6:e20476.
Humphrey-Murto S, Varpio L, Gonsalves C, Wood TJ. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017;39:14–9.
Havd GSL. Consensus measurement in Delphi studies Review and implications for future quality assurance. Technol Forecast Soc Change. 2012;79:1525–36.
Hasson F, Keeney S, McKenna HSL. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15.
The common clinical competency framework for non-medical ophthalmic healthcare professionals in secondary care. The Royal College of Ophthalmologists. 2016. https://www.rcophth.ac.uk/wp-content/uploads/2016/11/CCCF-Glaucoma.pdf.
Linstone HA, Turoff M. The Delphi Method: techniques and applications. s.l. : Reading, MA: Addison-Wesley Publishing Company; 1975.
Diamond Ivan, Grant Robert, Feldman Brian, Pencharz Paul, Ling Simon, Moore Aideen, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of delphi studies. J Clin Epidemiol. 2014;67:401–9.
Gracht HeikoAvonder. Consensus measurement in Delphi studies: Review and implications for future quality assurance Author links open overlay panel. Technol Forecast Soc Change. 2012;79:1525–36.
Adler M, Ziglio E. Gazing into the Oracle: the Delphi method and its application to social policy and public health. Bristol: Jessica Kingsley Publishers; 1996.
Lakhani BK, Giannouladis K, Leighton P, King AJ. Seeking a practical definition of stable glaucoma: a Delphi consensus survey of UK glaucoma consultants. Eye. 2019;34:335–43.
Campbell SE, Azuara-Blanco A, Campbell MK, Francis JJ, Greene AC, Ramsay CR, et al. Developing the specifications of an open angle glaucoma screening intervention in the United Kingdom: a Delphi approach. BMC Health Serv Res. 2012;12:447.
Lee PP, Sultan MB, Grunden JW, Cioffi GA.IOP Consensus Panel Assessing the importance of IOP variables in glaucoma using a modified delphi process. J Glaucoma. 2010;12:281–7.
Wilson MR, Lee PP, Weinreb RN, Lee BL, Singh K. A panel assessment of glaucoma management: modification of existing RAND-like methodology for consensus in ophthalmology. Part I: methodology and design. Am J Ophthalmol. 2008;145:570–74.
Ismail R, Azuara-Blanco A, Ramsay CR. Consensus on outcome measures for glaucoma effectiveness trials: results from a delphi and nominal group technique approaches. J Glaucoma. 2016;25:539–46.
Somner JEA, Ismail R, Froud R, Azuara-Blanco A, King AJsl. Consensus generation of a minimum set of outcome measures for auditing glaucoma surgery outcomes-a Delphi exercise. Graefes Arch Clin Exp Ophthalmol. 2018;256:2407–11.
Sii S, Barton K, Pasquale LR, Yamamoto T, King AJ, Azuara-Blanco A. Reporting harm in glaucoma surgical trials: systematic review and a consensus-derived new classification system. Am J Ophthalmol. 2018;194:153–62.
Kotecha A, Longstaff S, Azuara-Blanco A, Kirwan JF, Morgan JE, Spencer AF, et al. Developing standards for the development of glaucoma virtual clinics using a modified Delphi approach. Br J Ophthalmol. 2018;102:531–34.
Lee PP, Sultan MB, Grunden JW, Cioffi GA.IOP Consensus Panel Assessing the importance of IOP variables in glaucoma using a modified delphi process. J Glaucoma. 2010;19:281–7.
Azuara-Blanco A, Burr J, Thomas R, Maclennan G, McPherson S. The accuracy of accredited glaucoma optometrists in the diagnosis and treatment recommendation for glaucoma. Br J Ophthalmol. 2007;91:1639–43.
Banes MJ, Culham LE, Bunce C, Xing W, Viswanathan A, Garway-Heath D. Agreement between optometrists and ophthalmologists on clinical management decisions for patients with glaucoma. Br J Ophthalmol. 2006;90:579–85.
Marks JR, Harding AK, Harper RA, Williams E, Haque S, Spencer AF, et al. Agreement between specially trained and accredited optometrists and glaucoma specialist consultant ophthalmologists in their management of glaucoma patients. Eye. 2012;26:853–61.
Ho S, Vernon SA. Decision making in chronic glaucoma−optometrists vs ophthalmologists in a shared care service. Ophthalmic Physiol Opt. 2011;31:168–73.
Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al. Consensus development methods, and their use in clinical guideline development: a review. Health Technol Assess. 1998;2:37–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lakhani, B.K., Giannouladis, K., Leighton, P. et al. Defining stable glaucoma: a Delphi consensus survey of UK optometrists with a specialist interest in Glaucoma. Eye 35, 2524–2534 (2021). https://doi.org/10.1038/s41433-020-01251-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01251-w