Abstract
Purpose
The management of suprachoroidal haemorrhage (SCH) remains a challenge. We aimed to analyse and discuss the safety and efficacy outcomes of SCH drainage surgery over a 10-year period in one of the largest tertiary centres in the UK.
Methods:
Retrospective observational study of consecutive patients who underwent SCH drainage in Manchester Royal Eye Hospital over a 10-year period (from 2008 to 2018). Safety and efficacy were assessed by analysing surgery-related complications and functional and anatomical success. Outcomes of those who underwent external drainage alone versus combined drainage and vitrectomy were compared.
Results:
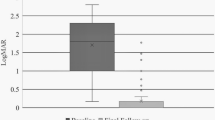
Twenty consecutive patients with a mean age of 70 ± 19 years were studied. Age over 70 years, hypertension, cardiovascular disease, and glaucoma were the most common risk factors for SCH. Eleven patients underwent external drainage alone and nine patients had combined vitrectomy and drainage. Overall, mean pre-operative BCVA improved from 2.22 ± 0.26 logMAR (20/3319 Snellen) to 1.42 ± 1.02 LogMAR (20/526 Snellen) at last follow-up visit (p = 0.002). Severe hypotony occurred in 4 patients. Overall anatomical and functional success rates were both 75%.
Conclusions
Drainage of SCH with or without vitrectomy is a valuable approach in the management of extensive SCH, a condition generally associated with poor prognosis.
Similar content being viewed by others
Introduction
Suprachoroidal haemorrhage (SCH) is one of the most devastating and sight-threatening ocular disorders [1,2,3]. It can be caused by trauma or as a complication of surgical procedures, including cataract surgery, glaucoma filtration surgery, keratoplasty, and vitreoretinal surgery [4,5,6,7]. A change in transluminal vascular pressure, caused by acute intraoperative hypotony, may cause choroidal effusion that can lead to rupture of the small arteries crossing the suprachoroidal space resulting in SCH [8].
Multiple risk factors are associated with SCH. Systemic risk factors include advanced age, systemic hypertension, diabetes mellitus, intraoperative tachycardia, arteriosclerosis, and coagulation disorders [9]. Ocular risk factors comprise of high myopia, glaucoma, previous vitrectomy, pseudophakia, and aphakia [10]. The reported incidence of SCH varies from 0.03 to 0.13% after phacoemulsification [2, 7] to 0.15% and 0.4–0.6% after filtration and vitreoretinal procedures, respectively [4, 5].
The literature regarding the surgical management of SCH is scarce, although it is uniformly reported as a condition with guarded functional outcomes [1, 3, 7]. Surgical management options of SCH range from external drainage alone to combined drainage and pars plana vitrectomy (PPV). The optimal approach is still a matter of debate and current practice varies depending on the clinical scenario and surgeon’s preference.
We present a large series of SCH drainage surgery and discuss its outcomes, with the purpose of building evidence on the safety and efficacy of the surgical options in SCH management.
Methods
This is a retrospective, non-randomised surgical series of patients attending a tertiary teaching hospital—Manchester Royal Eye Hospital, over a 10-year period (2008–2018). The study had approval from the local Institutional Review Board, adhered to the tenets of the Declaration of Helsinki and all patient data extracted were anonymised for analysis. The EQUATOR network PROCESS guidelines [11] were followed.
The clinical records of the patients who underwent surgical drainage of SCH over the study period were retrieved. A minimum follow-up time of 3 months was required. Patients’ demographic and baseline characteristics (age, gender, risk factors, and SCH aetiology), pre-operative data including best-corrected visual acuity (BCVA), clinical examination findings (lens status, SCH extent, macular involvement, combined vitreous haemorrhage, or retinal detachment), time until surgical intervention, and type of surgical intervention were assessed.
Indications for surgery were: kissing choroidal detachments, large non-kissing choroidal detachments with macular involvement, co-existent vitreous incarceration or haemorrhage, co-existent retinal incarceration or detachment, lens subluxation, high uncontrolled intraocular pressure (IOP), and/or intractable eye pain. In order to optimise surgical outcomes, serial ultrasounds were performed to look for evidence of liquefaction of SCH clots and the site of highest haemorrhage.
The decision to perform external drainage alone or a combined drainage and PPV was made according to surgeons’ preference and clinical indication (e.g., the co-existence of a retinal detachment or a dense vitreous haemorrhage implied a combined procedure). All surgeries were performed by experienced retinal surgeons and consisted of: (i) external drainage alone—either limited or 360-degree conjunctival peritomy was performed with isolation of the recti muscles depending on the site and number of quadrants to be drained. The conjunctiva and recti muscles were carefully handled in order to get good controlled access posteriorly. An anterior chamber maintainer with a high infusion pressure to encourage drainage was then placed. Full thickness 2–3 mm long scleral incisions were made in the areas of the highest choroidal detachment, as previously studied on ocular ultrasound. Pressure exerted on the eye facilitated drainage of the liquefied blood. The sclera was not sutured and the conjunctiva was closed with 8-0 vicryl sutures. Video 1 (supplementary material) demonstrates an example of the described technique.; (ii) combined drainage and PPV—external drainage as described above followed by 20 or 23-gauge PPV using anterior chamber maintainer at the start of the surgery switching to a pars plana infusion when safe to do so; the use of perfluorocarbon liquid (PFCL) and the choice of tamponade was made on a case by case basis at the surgeon’s discretion. Of note, in two cases, internal drainage was performed instead as a part of vitrectomy. In these cases the surgeon chose to first place an anterior chamber (AC) maintainer so as to prevent the risk of fluid flowing into the subretinal space. There was enough vitreous space to allow for placement of 23 G ports and carry out a PPV, and the SCH was drained internally through a pre-existing retinal break after creating a small break in the choroid. There is a risk of further choroidal bleeding using this approach, which was controlled using appropriate endodiathermy and a high infusion pressure. All cases were performed under general anaesthesia.
The following post-operative data were retrieved at last visit: BCVA, IOP, retinal status, surgery-related and other complications. The safety and efficacy (anatomical and functional) outcomes of patients who underwent external drainage surgery vs drainage and PPV were comparatively discussed. Criteria for success were defined as follows:
-
1.
Absolute anatomical success—achieving an attached retina at the posterior pole at last visit without tamponade in the absence of severe hypotony (IOP < 6 mmHg).
-
2.
Relative anatomical success—achieving an attached retina at the posterior pole at last visit with oil tamponade and in the absence of severe hypotony.
-
3.
Functional success—BCVA improvement of 2 LogMAR lines or more compared to baseline.
BCVA was collected as Snellen visual acuity and converted to LogMAR units for statistical analysis and non-numeric values were changed as described by Lee and colleagues [12]: counting fingers = 1.7 Log MAR, hand movement = 2.0 LogMAR, light perception = 2.3 LogMAR, and no light perception = 3.0 LogMAR. Frequencies and means (±standard deviation) are reported for qualitative and continuous variables, respectively. Descriptive statistics were performed using STATA v14, considering a p value < 0.05 for statistical significance.
Results
A total of 20 consecutive patients were included, with a mean age of 70 ± 19 (range: 15–93) years. Mean follow-up time was 19 ± 17 (range: 3–48) months. All eyes in this series had extensive SCH at presentation.
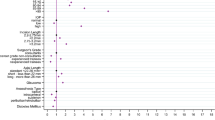
Of the 20 patients, eighteen resulted from complicated intraocular surgery: phacoemulsification (n = 7), corneal surgery (n = 5), glaucoma filtration procedure (n = 4), and vitreoretinal surgery (n = 2). Two cases occurred following blunt trauma resulting in globe rupture. Each of these cases underwent urgent primary globe repair and were diagnosed with massive SCH postoperatively. Systemic risk factors included age higher than 70 years, hypertension, and cardiovascular disease. Table 1 outlines the demographics, risk factors, timings, and other clinical data for each patient.
Of the seven patients with a SCH related to cataract surgery, five had a complicated procedure with posterior capsular rupture (four of them were left aphakic and one with a sulcus IOL), and two had a routine procedure with an IOL implanted in the bag. SCHs that developed in three of the four patients who had glaucoma surgery were in the post-operative period. These timings are summarised in Table 1. Our data did not show a difference in outcomes between post-operative and intraoperative development of the SCH.
The mean time from presentation until the surgical intervention was 11 ± 6 days (range 1–22 days) for all cases; 6–22 days after cataract surgery, 9–18 days following corneal surgery, 2–13 days after glaucoma surgery, 1–14 days after vitreoretinal surgery, and 6–10 days following primary globe repair for the trauma cases.
At initial examination with ocular ultrasound, 16 patients had four quadrants of SCH with macular involvement, one patient had three quadrants of SCH with macular involvement and three patients had 2 quadrants of choroidal apposition without macular involvement. Furthermore, seven of the 20 patients (35%) had vitreous haemorrhage and six of the 20 patients had retinal detachment at presentation.
Overall, mean pre-operative BCVA was 2.22 ± 0.26 LogMAR (20/3319 Snellen), improving to 1.42 ± 1.02 LogMAR (20/526 Snellen) at last follow-up visit (p = 0.002). Final mean IOP was 13.3 ± 5.3 mmHg. Hypotony occurred in 4 patients (20%). Of all cases, 11 patients (55%) underwent external drainage only and 9 patients (45%) had combined vitrectomy and drainage.
External drainage only group
The average time to intervene in the drainage only group was 12 ± 7 (range: 1–22) days. In the drainage only group the mean BCVA improved from 2.16 ± 0.16 LogMar (20/2891 Snellen) to 0.98 ± 0.94 LogMar (20/191 Snellen) (p = 0.001). The mean IOP at last visit was 14.3 ± 3.8 mmHg. BCVA improvement and absolute anatomical success were achieved in 9 out of 11 patients (81.8%). There were 2 failures; one patient (case 17) developed hypotony and a blind eye, and another one (case 6) needed further vitrectomy after developing a retinal detachment. Table 2 summarises the safety and efficacy outcomes of the patients who had drainage surgery only.
Combined drainage and PPV group
The remaining nine patients underwent a combined procedure. The average time to intervention was 10 ± 4 days (range: 3–18). The mean BCVA changed from 2.28 ± 0.35 LogMAR (20/3811 Snellen) to 1.96 ± 0.89 LogMAR (20/1824 Snellen) (p = 0.33). The mean IOP at last visit was 12 ± 7 mmHg. In this group, significant BCVA improvement and functional success was observed in six of the nine patients (67%). Absolute success was only achieved in four out of nine patients (44%), and a further relative success in two patients (22%), giving a total success of 66.7%. Surgical failure was seen in three out of nine patients.
Intraoperative perfluorocarbon was used in 5 (56%) of the cases. Two cases (22%) had vitrectomy with internal drainage. Overall, the preferred tamponade of choice was silicone oil (eight of the nine patients). One patient (case 15) had air tamponade—this case resulted in anatomical failure with four quadrants of SCH, involving the macula. For patients with absolute anatomical success, two went onto develop corneal decompensation. One patient had a penetrating keratoplasty four years after the SCH drainage surgery. The second patient was offered a DSAEK procedure however declined due to multiple procedures and risk involved.
Table 3 summarises the safety and efficacy outcomes of the patients who had combined pars plana vitrectomy and drainage surgery.
Comparison between groups
Mean BCVA improvement was higher in the drainage only group (1.18 ± 0.89 logMAR units) compared to the combined PPV+ drainage group (0.32 ± 0.9 logMAR units), p value = 0.047.
However, the proportion of patients in which functional success (BCVA improvement of more than 2 lines) was not statistically significantly different between groups: drainage only group (9/11 patients) versus drainage and PPV group (6/9 patients), p value = 0.44. Anatomical failure rate was also not different amongst the two groups—drainage only (2/11 patients) versus drainage and PPV (3/9 patients), p value = 0.44.
Discussion
SCH is a one of the most feared complications of intraocular surgery, and if left untreated, can result in complete loss of vision and phthisis bulbi. One study looking at seven eyes with SCH found that 41% had no light perception or light perception, 11% had counting fingers vision and 43% became phthisic or were eviscerated/enucleated during follow-up. There are limited studies looking into the surgical outcomes of SCH and few have compared different surgical approaches. Our study is one of the largest consecutive series that aims to present the surgical outcomes when intervening for massive SCH, and comparing the two approaches: external transcleral drainage alone and external drainage combined with PPV.
Before planning a surgical procedure, careful consideration of all risk factors and avoiding intraoperative hypotony are the key principles in prevention of a significant SCH [13]. In our study the two most common systemic risk factors were age over 70 (60%) and hypertension (60%), followed by cardiovascular disease (43%). The two most common ocular risk factors were glaucoma (30%) and high myopia (20%).
The first surgical dilemma once the SCH develops is whether to intervene surgically or not. It has been previously recommended that surgical intervention is required in all cases where the macula is affected by the haemorrhage, and in those eyes where the SCH extends to more than 2 quadrants posterior to the equator without involvement of the macula [3]. Limited SCH, with no vitreous or retinal incarceration or macular involvement, can be monitored [14]. We agree that the only definite indication to intervene surgically is the involvement of macula in the SCH, since the foveal photoreceptors can be affected by toxicity or hypoxia due to the underlying SCH [1, 13]. If the macula is not involved, a conservative approach of monitoring the eye with serial ultrasonography may be sensible. The main indications for intervening in our series were a SCH with choroidal detachment involving the posterior pole, kissing choroidal detachments, combined SCH and retinal detachment, and combined SCH and vitreous incarceration.
The optimal timing for surgical treatment of SCH has not been defined [9]. For a successful drainage of blood, it is essential that the blood has liquefied to allow it to flow smoothly. Animal models have suggested an interval of 1–2 weeks to allow for the haemolysis of suprachoroidal haemorrhage [15]. Previous studies have used the same time interval for a successful drainage [1]. In our study, the average time from the initial consultation to the day of surgical intervention was 11.1 days, which is comparable to other studies. Three patients had unsuccessful external drainage attempt in the first three days due to clotted blood (cases 13, 16, 17); However, waiting for longer intervals should be balanced against the poor outcomes associated with long-duration choroidal apposition [3, 16]. Of note, in our series, cases 5 and 6 had drainage after 22 and 20 days, respectively, with a satisfactory anatomical and functional outcome.
The optimal procedure for the secondary surgical management of SCH depends on the extent of the posterior segment damage. We suggest dividing SCH into 2 categories: (i) uncomplicated SCH, and (ii) SCH complicated by vitreous and retinal involvement. We believe that if there is uncomplicated SCH even if it is massive, external trans-scleral drainage is enough for achieving anatomical and functional improvement, without the morbidity associated with a more complex procedure. However, if there is vitreous haemorrhage or incarceration, external drainage should be combined with vitrectomy due to a higher risk of development of retinal tears and rhegmatogenous retinal detachment [3, 14].
External drainage
In cases of extensive uncomplicated SCH, good anatomical outcomes can be achieved with external trans-scleral drainage alone [16]. Trans-scleral drainage surgery is technically easier to perform than a PPV, in these unstable eyes with massive SCH. The aim of this procedure is to drain enough blood to flatten the posterior pole and not necessarily to completely drain all the SCH.
The external drainage group had a high absolute anatomical success in our study of 81.8% (9 out of 11 patients). A total of eoght patients in this cohort were found to have uncomplicated SCH, seven out of these achieved an improvement in vision and anatomical success following the procedure—Table 2. This clearly demonstrates that despite the extent of SCH, a simpler external drainage approach without any vitrectomy and internal tamponade may just be enough to achieve excellent anatomical and functional outcomes in such cases.
Combined drainage and vitrectomy group
At presentation, these cases were inherently more complex in nature than those in the drainage only group. Also, the surgical management of SCH was technically more challenging in this group due to the presence of either lens subluxation, and vitreous or retinal incarceration in an already unstable eye. Table 3 details pre-operative patients characteristics and indications for surgery. Our study demonstrates that despite the complexity of the surgery, functional improvement in vision was achievable, with anatomical and functional success seen in 67% (6/9) of cases.
In this subgroup of patients where vitrectomy was carried out, two cases had successful internal drainage of the SCH during vitrectomy, which is an uncommon approach. Both cases were secondary to trauma and required silicone oil tamponade to prevent rebleeding and retinal detachment. This can be an option especially in trauma cases where external drainage may be more challenging due to extensive scleral damage.
Our study is one of the largest series on surgical management of SCH. It adds to the limited evidence available on this condition. However, our results should be considered in line with expected study limitations. Firstly, as an uncommon condition, the non-randomised design and relatively small sample size limits drawing definite conclusions. Moreover, it can be challenging to compare the outcomes of groups of patients that are heterogeneous in nature. We studied patients from a single centre which, although is a large tertiary unit, emphasizes the importance of collaborative networking to increase the power and external validity of future studies.
In conclusion, the surgical management of massive SCH remains challenging, with the prognosis greatly depending on the severity of the damage at the presentation and pre-existing pathology. We recommend dividing SCH into two groups: uncomplicated SCH where trans-scleral drainage is usually successful; and the more complex SCH with vitreoretinal involvement where external drainage should be combined with vitrectomy with the use of silicone oil tamponade. Meticulous planning and choosing the appropriate surgical approach in managing SCH with all its complexities can result in favourable anatomical and functional outcomes in such vision-threatening cases.
Summary
What was known before
-
Suprachoroidal haemorrhage (SCH) is a sight-threatening condition with a guarded visual prognosis.
What this study adds
-
We present the functional and anatomical outcomes of a 10-year series of patients who underwent external drainage surgery with and without vitrectomy for SCH.
References
Laube T, Brockmann C, Bornfeld N. Massive suprachoroidal hemorrhage: Surgical management and outcome. GMS Ophthalmol Cases. 2015;5. https://doi.org/10.3205/oc000032
Eriksson A, Koranyi G, Seregard S, Philipson B. Risk of acute suprachoroidal hemorrhage with phacoemulsification. J Cataract Refract Surg. 1998;24:793–800.
Meier P, Wiedemann P. Massive suprachoroidal hemorrhage: secondary treatment and outcome. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2000;238:28–32.
Stein JD, Zacks DN, Grossman D, Grabe H, Johnson MW, Sloan FA. Adverse events after pars plana vitrectomy among medicare beneficiaries. Arch Ophthalmol Chic Ill 1960. 2009;127:1656–63. https://doi.org/10.1001/archophthalmol.2009.300
Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol Chic Ill 1960. 1986;104:201–5.
Obuchowska I, Mariak Z. Risk factors of massive suprachoroidal hemorrhage during extracapsular cataract extraction surgery. Eur J Ophthalmol. 2005;15:712–7.
Ling R. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478–80. https://doi.org/10.1136/bjo.2003.026138
Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy. Ophthalmology. 2014;121:311–7. https://doi.org/10.1016/j.ophtha.2013.06.021
Lavinsky F, Moisseiev J, Levkovitch-Verbin H. The surgical management of massive intraoperative and postoperative suprachoroidal hemorrhage: anatomic and functional outcomes. Arq Bras Oftalmol. 2013;76:212–4.
Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98:202–9.
Agha RA, Borrelli MR, Farwana R, Koshy K, Orgill D, et al. The PROCESS 2018 statement: Updating Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int J Surg Lond Engl. 2018;60:279–82. https://doi.org/10.1016/j.ijsu.2018.10.031
Lee JWY, Lai JSM, Yick DWF, Tse RKK. Retrospective case series on the long-term visual and intraocular pressure outcomes of phacomorphic glaucoma. Eye Lond Engl. 2010;24:1675–80. https://doi.org/10.1038/eye.2010.108
Kuhn F, Morris R, Mester V. Choroidal detachment and expulsive choroidal hemorrhage. Ophthalmol Clin N. Am. 2001;14:639–50.
Reynolds MG, Haimovici R, Flynn HW, DiBernardo C, Byrne SF, Feuer W. Suprachoroidal hemorrhage. Clinical features and results of secondary surgical management. Ophthalmology. 1993;100:460–5.
Lakhanpal V. Experimental and clinical observations on massive suprachoroidal hemorrhage. Trans Am Ophthalmol Soc. 1993;91:545–652.
Scott IU, Flynn HW, Schiffman J, Smiddy WE, Murray TG, Ehlies F. Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology. 1997;104:2039–46.
Acknowledgements
This research was facilitated by the Greater Manchester Local Clinical Research Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Qureshi, A., Jalil, A., Sousa, D.C. et al. Outcomes of suprachoroidal haemorrhage drainage with and without vitrectomy: a 10-year study. Eye 35, 1879–1885 (2021). https://doi.org/10.1038/s41433-020-01170-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01170-w
This article is cited by
-
The Royal College of Ophthalmologists’ National Ophthalmology Database Study of Cataract Surgery: Report 12, Risk factors for suprachoroidal haemorrhage during cataract surgery
Eye (2023)
-
Clinical characteristics and mortality rates for suprachoroidal hemorrhage: seven-year experience at a tertiary eye center
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)