Abstract
Purpose
To study features of Indocyanine green angiography (ICGA) in patients with presumed intraocular tuberculosis.
Methods
Retrospective study of 48 consecutive patients (77 eyes) who underwent ICGA. The following signs were analysed: choroidal perfusion inhomogeneity, early hyperfluorescent stromal vessels, round or oval hypofluorescent dark dots (HDDs), hypofluorescent geographic lesions (HGLs), fuzzy or lost pattern of large stromal choroidal vessels, disc hyperfluorescence and diffuse late choroidal hyperfluorescence.
Results
Among 44 eyes of 29 patients with no clinical evidence of choroidal involvement, only 7 eyes of 6 patients had no ICGA evidence of choroidal involvement. On the other hand, ICGA findings suggesting choroidal involvement were noted in 37 (84.1%) eyes of 23 patients in the form of HDDs in all 37 (100%) eyes, HGLs in 7 (18.9%) eyes, disc hyperfluorescence in 20 (45.5%) eyes, fuzzy stromal vessels in 17 (38.6%) eyes, early hyperfluorescent stromal vessels in 13 (29.5%) eyes, late pinpoint hyperfluorescence in 11 (25%) eyes and late diffuse choroidal hyperfluorescence in 7 (15.9%) eyes. Among 33 eyes of 19 patients with clinically evident choroidal involvement, the following findings were identified; HDDs in 12 (36.4%) eyes, HGLs in 10 (30.3%) eyes, both HDDs and HGLs in 9 (27.3%) eyes, disc hyperfluorescence in 11 (33.3%) eyes, early hyperfluorescent stromal vessels in 7 (21.2%) eyes, fuzzy stromal vessels in 6 (18.2%) eyes and late diffuse choroidal hyperfluorescence was present in 2 (6.1%) eyes.
Conclusions
ICGA is necessary in identifying and diagnosing subclinical tuberculous choroidal involvement. The most prevalent ICGA finding was persistent HDDs.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is a slowly progressive chronic, granulomatous infection caused by Mycobacterium tuberculosis [1]. TB usually affects the lungs, but can also affect other organs and systems like the cardiovascular system, genitourinary tract, gastrointestinal system, musculoskeletal system, central nervous system, skin, and eyes [2, 3].
Clinically, presumed intraocular tuberculosis (PIOTB) can be due to direct infection, or an indirect immune-mediated hypersensitivity response to mycobacterial antigens when there is no defined active systemic lesion anywhere in the body or the lesion is believed to be inactive [4,5,6,7,8]. Clinical manifestations of PIOTB include acute anterior uveitis, chronic granulomatous anterior uveitis, intermediate uveitis, vitritis, retinal vasculitis, neuroretinitis, solitary or multiple choroidal tubercles, multifocal choroiditis (MFC), choroidal granulomas, serpiginous-like choroiditis, subretinal abscess, endophthalmitis and panophthalmitis. Of these various intraocular presentations, the most common clinical presentations appear to be panuveitis and posterior uveitis [9, 10].
The diagnosis of tuberculous uveitis is usually presumptive. In most studies, the diagnostic criteria for presumed tuberculous uveitis were: (1) ocular findings consistent with possible intraocular TB with no other cause of uveitis suggested by history of symptoms or ancillary testing. (2) Strongly positive tuberculin skin test results (15 mm or more area of induration/necrosis). (3) Response to anti-tuberculous therapy (ATT) with absence of recurrences [4, 6, 7, 11,12,13,14,15,16]. The absence of clinically evident pulmonary TB does not rule out the possibility of ocular TB, as about 60% of patients with extrapulmonary TB have no evidence of pulmonary TB [17].
Indocyanine green angiography (ICGA) allows visualisation of the choroidal vessels including the choriocapillaris and stromal vessels as well as the choroidal stroma [18,19,20,21]. This is due to the fact that the dye fluoresces in the near infrared wavelength [22]. Indocyanine green molecule is twice as large as the fluorescein molecule (751 Daltons) but still is a very small molecule. The fact that it is almost completely (98–99%) protein bound results in a macromolecular behaviour (66,500 Daltons and larger). Thus, it does not leak from normal and moderately inflamed retinal vessels, but leaks through the large fenestrations of choriocapillaris, albeit slowly [18, 23].
Previous studies reported ICGA findings in PIOTB [15, 24,25,26,27,28]. The objective of this study is to investigate ICGA findings in a large series of patients with PIOTB uveitis who presented at our tertiary eye care centre.
Methods
The medical records of all patients who presented with PIOTB to the emergency room, King Abdulaziz University Hospital, Riyadh, Saudi Arabia from 2011 to 2019 were retrospectively reviewed. Patients were included in the study if they fulfil all the following inclusion criteria consistent with the diagnosis of PIOTB: ocular findings consistent with possible intraocular TB with no other cause of uveitis suggested by history of symptoms or ancillary testing, strongly positive purified protein derivative skin test results (15 mm or more area of induration/necrosis), and response to ATT with absence of recurrences after completion of treatment [13, 15, 16]. None of the patients included in the study underwent interferon gamma release assay as the test was not available in our institute until recently. All patients were managed and followed-up by one of the authors (AMA). Charts were reviewed for demographic data (age and gender), time interval between onset of symptoms and presentation to our institute, initial best-corrected visual acuity, findings of slit-lamp examination, and dilated fundus examination. All patients underwent imaging using optical coherence tomography, fundus photography (colour and red-free), fluorescein angiography (FA), and ICGA using the Heidelberg scanning laser ophthalmoscope (HRA-2; Heidelberg Engineering, Heidelberg, Germany). Only patients who had good quality ICGA images that can be evaluated were included. All patients gave informed and written consent. A bolus injection of 50 mg of indocyanine green diluted in 5-ml normal saline was injected within 5 s. Frames were captured at close intervals in the first 3 min, followed by intermediate phase frames at 7–15 min and late frames at 20, 25 and 30 min. Each ICGA was interpreted by two independent observers (MAA, LDS). In case of doubt, both observers, along with (AMA) reviewed the images together and reached a consensus.
ICGA signs
Based on previous publications [15, 20, 24,25,26,27, 29, 30], we looked at the following major ICGA signs of choroidal involvement: hypofluorescent dark dots (HDDs); both transient and persistent, hypofluorescent geographic lesions (HGLs), fuzzy vascular patterns of large stromal vessels and disc hyperfluorescence. Other minor ICGA signs that were previously reported were: disturbance/delay in early choriocapillaris circulation, hyperfluorescent pinpoints and diffuse late hyperfluorescence.
Statistical analysis
Data were collected and entered using Microsoft Excel 2018® database. Frequencies and percentages were calculated for each category. Cohen’s Kappa analysis was performed using SPSS® version 19.0 (IBM Inc., Chicago, Illinois) to examine inter-observer agreement between MAA and LDS. Kappa (κ) values were interpreted as follows: <0, poor agreement; 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement and >0.80, almost perfect agreement.
Results
Patient characteristics
A total of 48 patients (79 eyes) with PIOTB who underwent ICGA imaging were enroled in this study. Two eyes of two patients were excluded due to poor image quality. Therefore, good quality ICGA was available for 77 eyes of 48 patients. One eye was involved in 19 (39.6%) patients, and both eyes were involved in 29 (60.4%) patients. Table 1 presents the demographic and clinical characteristics of the study subjects.
Anterior segment findings were as follows; 33 (42.9%) eyes had granulomatous reaction in the form of mutton-fat keratic precipitates in 24 (31.2%) eyes, iris nodules in 2 (2.6%) eyes, both mutton-fat keratic precipitates and iris nodules in 7 (9.1%) eyes, 39 (50.6%) eyes had 1+ cells or less in the anterior chamber, 15 (19.5%) eyes had 2+ cells or more and 21 (27.3%) eyes had posterior synechia.
Posterior segment findings were as follows; vitreous involvement in the form of vitreous haze in 37 (48.1%) eyes, large vitreous snowballs in 23 (29.9%) eyes and macular oedema in the form of cystoid spaces, diffuse retinal thickening, and/or subretinal fluid was identified in 40 (51.9%) eyes. Retinal vasculitis manifesting as perivenous vascular sheathing was noted in 12 (15.6%) eyes. Clinically evident choroidal involvement was present in 33 (42.9%) eyes (19 patients; bilateral disease in 14 patients and unilateral disease in 5 patients) in the form of active MFC in 21 (27.3%) eyes, serpiginous-like choroiditis in 10 (12.9%) eyes and solitary choroidal granuloma in 2 (2.6%) eyes. Optic nerve head hyperaemia was detected in 37 (48.1%) eyes. Forty-four (57.1%) eyes (29 patients; bilateral disease in 15 patients and unilateral disease in 14 patients) did not show clinical or FA evidence of choroidal involvement. According to the Standardisation of Uveitis Nomenclature [31], the anatomic diagnosis was intermediate uveitis in 26 (33.8%) eyes, posterior uveitis in 27 (35.1%) eyes and panuveitis in 24 (31.2%) eyes.
Based on our protocol [13], all patients with PIOTB received standard treatment with anti-tuberculous medication for 9 months starting with four drugs in the first 2 months (isoniazid 300 mg daily, rifampicin 450 mg daily, pyrazinamide 30 mg daily and ethambutol 15 mg/kg daily), followed by a continuation phase with isoniazid and rifampicin only. Systemic corticosteroids (1 mg/kg) and pyridoxine were started along with anti-tuberculous medication to all patients. The follow-up was every 2 weeks for 8 weeks and then every 1–2 months as required to monitor response to therapy. None of the patients developed flare-up after discontinuation of anti-tuberculous treatment.
ICGA findings
ICGA was evaluated in 77 eyes (48 patients) included in this study. Inter-observer agreement with regard to detecting different ICGA signs was as follows: all HDDs, κ = 0.674; persistent HDD, κ = 0.792; transient HDD, κ = 0.698; HGLs, κ = 0.941; fuzzy vascular patterns, κ = 0.280 and disc hyperfluorescence, κ = 0.946. ICGA signs of choroidal involvement was present in 70 (90.9%) eyes (42 patients; 87.5%).
ICGA findings in eyes with no clinical evidence of choroidal involvement
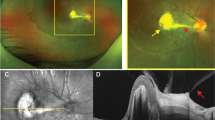
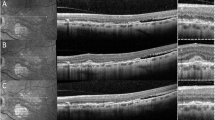
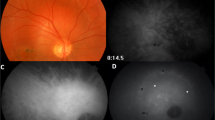
Among the 44 eyes (29 patients) with no clinical or FA evidence of choroidal involvement, 37 (84.1%) eyes of 23 patients had ICGA findings suggestive of choroidal involvement (bilateral disease in 14 patients and unilateral disease in 9 patients). The ICGA signs were HDDs in all 37 (100%) eyes (Fig. 1). Among these eyes, 23 (62.2%) eyes of 13 patients had persistent HDDs up to the late frames, and 14 (37.8%) eyes of 10 patients had partial thickness HDDs that became isofluorescent on the late angiographic frames. Seven eyes (18.9%) of 4 patients had HGLs in addition to HDDs.
Disc hyperfluorescence was seen in 20 (45.5%) eyes (13 patients), It has to be noted that on ICGA the disc should be completely dark and any fluorescence present is abnormal and is termed disc hyperfluorescence [30]. Early hyperfluorescent stromal choroidal vessels were noted in 13 (29.5%) eyes (nine patients). Fuzzy or lost pattern of choroidal stromal vessels were seen in 17 (38.6%) eyes (11 patients). Late diffuse choroidal hyperfluorescence was seen in seven (15.9%) eyes (four patients). Late pinpoint hyperfluorescence was detected in 11 (25%) eyes (eight patients).
Furthermore, among eyes with no clinically evident choroidal involvement that had ICGA signs (n = 37), the anatomic diagnosis was intermediate uveitis in 18 (48.6%) eyes (24 patients), panuveitis in 12 (32.4%) eyes (8 patients) and posterior uveitis in 7 (18.9%) eyes (5 patients). In this group, macular oedema was diagnosed in 24 (64.9%) eyes of 16 patients. Among the seven eyes (six patients) with no clinical and ICGA findings of choroidal involvement, six (85.7%) eyes of six patients had macular oedema.
ICGA findings in eyes with clinically evident choroidal involvement
Within the 33 eyes of 19 patients with clinically evident choroidal involvement, 10 (30.3%) eyes of 7 patients had serpiginous-like choroiditis in which ICGA showed HGLs in all (100%) eyes which was accompanied with HDDs in 5 of the 10 eyes (50%) (3 patients) (Fig. 2). Among the 21 (63.6%) eyes of 15 patients that had a clinical diagnosis of MFC, ICGA revealed HDDs in 12 (57.1%) eyes (9 patients) (Fig. 3), HGLs in 5 (23.8%) eyes (3 patients) (Fig. 4) and both HDDs and HGLs in 4 (19.1%) eyes (3 patients) (Fig. 3). Two (6.1%) eyes of two patients had solitary choroidal granuloma which appeared on ICGA as a large area of hypofluoresence that persisted throughout the study (Fig. 5). In addition, HDDs were noted in one of the two eyes with solitary choroidal granuloma.
Other ICGA signs noted were disc hyperfluorescence in 11 (33.3%) eyes (nine patients), early hyperfluorescent stromal choroidal vessels in 7 (21.2%) eyes (seven patients), fuzzy or lost pattern of choroidal stromal vessels in 6 (18.2%) eyes (six patients), late diffuse choroidal hyperfluorescence in 2 (6.1%) eyes (two patients) and late pinpoint hyperfluorescence in 4 (12.1%) eyes (four patients). In this group of patients, macular oedema was diagnosed in ten (30%) eyes of eight patients.
Discussion
We report ICGA findings of PIOTB in a series of 77 consecutive eyes. To the best of our knowledge, this is the largest systematic study of ICGA findings in PIOTB. Indocyanine green dye has high affinity to larger proteins, and fluoresces at near infrared wavelengths. This allows visualisation beyond the retinal pigment epithelium, which will help in detecting subclinical choroidal involvement that is not evident by fundus examination or FA. This has proved to be of high importance in follow-up to detect subclinical disease activity that needs proper attention and appropriate management [18, 29]. In the current study, ICGA allowed us to diagnose subclinical choroidal involvement in 84.1% of eyes that had no clinical or FA evidence of choroidal involvement.
Similarly, Wolfensberger et al. reported a series of 15 eyes of 8 patients in which ICGA disclosed subclinical choroidal lesions that were not visible clinically nor on fundus FA, and concluded that ICGA was useful in assessing and quantifying the unknown extent of choroidal involvement in PIOTB [15]. Massy and Herbort [28] found in a retrospective review of 54 eyes of 38 patients with PIOTB that there was a preferential involvement of the choroid for which ICGA was the preferred tool of investigation. Thus, HDDs in patients with no clinical evidence of choroidal involvement could represent subclinical tuberculous choroidal infiltrates. These choroidal infiltrates could be either full thickness (HDDs persistent up to late angiographic frames) or partial thickness (HDDs becoming iso-fluorescent on late angiographic frames) [15, 27]. Therefore, ICGA should be considered in eyes diagnosed to have PIOTB without clinical evidence of choroidal involvement.
We noted that HDDs were the most common, consistent and reliable ICGA sign (60 out of 77 eyes) (77.9%)) with an excellent inter-observer agreement. Furthermore, HGLs were found in all ten eyes with serpiginous-like choroiditis. HGLs were also noted in 8 out of 24 eyes with MFC. They were also found in seven eyes with no clinically evident choroidal involvement. This hypofluorescent geographic distribution was described initially by De Luigi et al. [24] in a case report of a multifocal serpiginous-like choroiditis and attributed the hypofluoresence to choriocapillaritis instead of choroiditis. When this pattern is formed by multiple confluent lesions, a serpiginous-like picture can be observed.
The second most common sign was disc hyperfluorescence (40.3%), which included all cases where the disc was not dark in the intermediate-late phase as it should normally be. This was seen in a slightly higher proportion of cases than what was reported in previous series [28]. Early hyperfluorescent stromal vessels in ICGA that was seen in only 25% was much less than what was reported by Massy and Herbort, but late pinpoint hyperfluorescence had a similar rate (20%) [28]. These pinpoint hyperfluorescent spots are thought to be due to extensive binding of ICG molecules in areas where granulomatous foci developed, and they are well-documented in sarcoidosis and Vogt–Koyanagi–Harada disease which are other granulomatous uveitic entities [29, 30]. These hyperfluorescent pinpoints tended to respond to treatment [29, 30]. Fuzzy vessels correspond to active diffuse inflammatory vasculopathy leading to leakage from these inflamed vessel walls, leading to the late diffuse choroidal hyperfluorescence [15]. These signs were found to be reversible almost completely in response to treatment [15]. Fuzzy choroidal vessels were not as frequent as what was reported in previous studies [15, 28]. Massy and Herbort [28] reported that it was the most frequent sign in PIOTB. It is possible that fuzzy choroidal vessels might be only picked up by more experienced ICGA readers as evident by the low κ value.
In conclusion, we report the ICGA findings in a large consecutive group of PIOTB. The most consistent findings were HDDs of variable size, and disc hyperfluorescence. ICGA angiography must be considered in all PIOTB patients in order to diagnose subclinical choroidal involvement.
Summary
What was known before
-
ICGA is crucial in detecting subclinical choroidal involvement leading to early diagnosis in patients with uveitis.
-
ICGA is the method of choice to monitor disease activity in uveitic entities involving the choroid.
What this study adds
-
This study describe indocyanine green angiographic findings in a large series of 48 patients (77 eyes) with presumed intraocular TB.
-
ICGA was mandatory in identifying and diagnosing subclinical tuberculous choroidal involvement.
References
Centers for Disease Control (USA). Tuberculosis—data and statistics 2015. http://www.cdc.gov/tb/statistics/default.htm.
Glassroth J, Robins AG, Snider DE. Tuberculosis in the 1980s. N Engl J Med. 1980;302:1441–50.
Baydur A. The spectrum of extrapulmonary tuberculosis. West J Med. 1977;126:253–62.
Rosen PH, Spalton DJ, Graham EM. Intraocular tuberculosis. Eye. 1990;4:486–92.
Helm CJ, Holland GN. Ocular tuberculosis. Surv Ophthalmol. 1993;38:229–56.
Sheu SJ, Shyu JS, Chen LM, Chen YY, Chirn SC, Wang JS. Ocular manifestations of tuberculosis. Ophthalmology. 2001;108:1580–5.
Sakai J, Matsuzawa S, Usui M, Yano I. New diagnostic approach for ocular tuberculosis by ELISA using the cord factor as antigen. Br J Ophthalmol. 2001;85:130–3.
Biswas J, Badrinath SS. Ocular morbidity in patients with active systemic tuberculosis. Int Ophthalmol. 1995;19: 293–8.
Al-Mezaine HS, Al-Muammar A, Kangave D, Abu El-Asrar. Clinical and optical coherence tomographic findings and outcome of treatment in patients with presumed tuberculous uveitis. Int Ophthalmol. 2008;28:413–23.
Al Dhahri H, Al Rubaie K, Hemachandran S, Mousa A, Gikandi PW, Al-Mezaine HS, et al. Patterns of Uveitis in a University-based Tertiary Referral Center in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2015;23:311–9.
Sarvananthan N, Wiselka M, Bibby K. Intraocular tuberculosis without detectable systemic infection. Arch Ophthalmol (Chic, Ill 1960). 1998;116:1386–8.
Cimino L, Herbort CP, Aldigeri R, Salvarani C, Boiardi L. Tuberculous uveitis, a resurgent and underdiagnosed disease. Int Ophthalmol. 2009;29:67–74.
Al-Qarni A, Abouammoh MA, Almousa AN, Mousa A, Abu El-Asrar AM. Presumed tuberculous uveitis in a university-based tertiary referral center in Saudi Arabia. Int Ophthalmol. 2019;39:317–33.
Morimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A, et al. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002;109:851–7.
Wolfensberger TJ, Piguet B, Herbort CP. Indocyanine green angiographic features in tuberculous chorioretinitis. Am J Ophthalmol. 1999;127:350–3.
Abu El-asrar AM, Abouammoh M, Al-Mezaine HS. Tuberculous uveitis. Int Ophthalmol Clin. 2006;50:19–39.
Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63:25–55.
Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology. 1998;105:432–40.
Kuhn D, Meunier I, Soubrane G, Coscas G. Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachments. Arch Ophthalmol (Chic, Ill 1960). 1995;113:1392–8.
Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;06:1928–34.
Chang AA, Morse LS, Handa JT, Morales RB, Tucker R, Hjelmeland L, et al. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology. 1998;105:1060–8.
da Silva FT, Hirata CE, Sakata VM, Olivalves E, Preti R, Pimentel SL, et al. Indocyanine green angiography findings in patients with long-standing Vogt-Koyanagi-Harada disease: a cross-sectional study. BMC Ophthalmol. 2012;12:40.
Baker KJ. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc Soc Exp Biol Med. 1966;122:957–63.
De Luigi G, Mantovani A, Papadia M, Herbort CP. Tuberculosis-related choriocapillaritis (multifocal-serpiginous choroiditis): follow-up and precise monitoring of therapy by indocyanine green angiography. Int Ophthalmol. 2012;32:55–60.
Papadia M, Herbort CP. Unilateral papillitis, the tip of the iceberg of bilateral ICGA-detected tuberculous choroiditis. Ocul Immunol Inflamm. 2011;19:124–6.
Knecht PB, Papadia M, Herbort CP. Secondary choriocapillaritis in infectious chorioretinitis. Acta Ophthalmol. 2013;91:e550–5.
Milea D, Fardeau C, Lumbroso L, Similowski T, Lehoang P. Indocyanine green angiography in choroidal tuberculomas. Br J Ophthalmol. 1999;83:753.
Massy R, Herbort CP. Contribution of dual fluorescein and indocyanine green angiography to the appraisal of presumed tuberculous Chorioretinitis in a non-endemic area. J Ophthalmic Vis Res. 2017;12:30–38.
Wolfensberger TJ, Herbort CP. Indocyanine green angiographic features in ocular sarcoidosis. Ophthalmology. 1999;106:285–9.
Abouammoh MA, Gupta V, Hemachandran S, Herbort CP, Abu El-Asrar AM. Indocyanine green angiographic findings in initial-onset acute Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2016;94:573–8.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Acknowledgements
The authors thank Ms Connie B. Unisa-Marfil for secretarial assistance. This work was supported by King Saud University through Vice Deanship of Research Chair, Dr Nasser Al Rashid Research Chair in Ophthalmology (AMAE-A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abouammoh, M.A., De Simone, L., Almousa, A.N. et al. Indocyanine green angiographic findings in presumed intraocular tuberculosis. Eye 35, 1680–1687 (2021). https://doi.org/10.1038/s41433-020-01144-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01144-y
This article is cited by
-
Tuberculosis reactivation demonstrated by choroiditis and inflammatory choroidal neovascular membrane in a patient treated with immune checkpoint inhibitors for malignant mucosal melanoma
Journal of Ophthalmic Inflammation and Infection (2023)