Abstract
Background and aims
Decreased heart-rate-variability (HRV) indicates increased sympathetic nervous system (SNS) activity and modulation with a shift in the sympatho-vagal balance towards SNS predominance. Increased SNS activity may precede volume-loading hypertension, contribute to increases in cardiac troponin T (cTnT), endothelial dysfunction and small vessel disease. Therefore, we investigated the retinal vasculature, HRV during flicker-light-induced-provocation (FLIP) and systemic cTnT, a marker of cardiac stress, to provide further evidence in support of the brain–retina–heart link.
Methods
Cross-sectional observations were obtained from a bi-ethnic cohort (N = 264), aged 23–68 years. Fasting serum samples for cTnT were obtained. Retinal vascular calibres were quantified from mydriatic eye fundus images and dynamic retinal vessel calibre responses were determined during FLIP. Time-and frequency domain parameters of HRV were calculated during FLIP for each participant.
Results
Africans had wider venules and attenuated time domain parameters during FLIP. In Africans, inverse associations emerged between arteriolar dilation and both cTnT and root-mean squared of the standard deviations of successive RR-intervals (rMSSD) (p = 0.030), and between arteriolar constriction and both low-frequency expressed in normalised units (LFnu) (p = 0.003) and high-frequency expressed in normalised units (p = 0.021). Wider venules inversely associated with standard deviation of the NN intervals (SDNN) as well as LFnu (p = 0.009) in Africans. An opposite profile was observed in Caucasians with both time-and frequency domain parameters of HRV in relation to retinal vessel structure and function.
Conclusion
FLIP elicited increased SNS activity and modulation in this bi-ethnic cohort. In Africans, decreased HRV during FLIP accompanied arteriolar and venular responses and elevated systemic levels of cTnT, implying that the SNS exerted a significant effect on the smooth muscle tone of the retinal vasculature. Disrupted retinal autoregulation may imply general autonomic nervous system dysfunction; exemplifying central control by the brain on all systemic regulatory functions, across different vascular beds.
Similar content being viewed by others
Introduction
Controversy exists regarding the contribution of the autonomic nervous system (ANS) to the retinal microvasculature’s autoregulatory capacities [1,2,3]. Lanigan et al. demonstrated that mean retinal vessel responses to systemic sympathetic stimulation were significantly reduced in sympathectomised eyes [3]. Decreased sympathetic nervous system (SNS) may therefore cause loss of tone and/or altered haemodynamics of all microvascular beds and such decreased SNS activity may occur as an integral feature of a general ANS dysfunction or dysregulation. Indeed, peripheral autonomic neuropathy was linked to the regulatory impairment of the retinal neurovascular complex, leading to altered retinal vessel responses [4]. However, whether ANS alterations influence retinal vessel responses during flicker-light-induced-provocation (FLIP) remains unclear. The ability of the ANS to make rapid adjustments to situational demands is evident in the control of heart rate.
Heart-rate-variability (HRV) is the most often employed, non-invasive assessment of ANS activity and modulation. HRV is the variation in the time interval between successive heart beats [5]. Reduced HRV indicates a shift in the sympatho-vagal balance towards SNS predominance [6]. This may be attributed to increased SNS activity/modulation, vagal withdrawal or both [6, 7]. A decrease in cardiac vagal tone accompanied impaired coronary microvascular function [6], a decrease in resting cerebral blood flow and reduced metabolic activity [8]. Reduced HRV has also been observed in Africans during acute mental stress, although not in Caucasians [9, 10]. Reduced resting HRV was also observed in Afro-Americans compared to European Americans [8].
The SNS is directly activated in myocardial ischemia and small vessel diseases, but increased SNS activity also precedes these events [11]. Higher levels of the cardiac stress marker cardiac troponin T (cTnT) was linked to endothelial and microvasculature disturbances in hypertensive patients [12]. Increases in cTnT levels in Africans in response to acute mental stress was observed, compared to Caucasians [9]. Such acute stress induced increases in cTnT might indicate a link between the SNS and cTnT release, as a link between cTnT and retinal vessel responses was recently established [13].
To our knowledge, no study has examined the time-and frequency domain measures of HRV during FLIP in relation to dynamic changes in the retinal vasculature and cTnT. We investigated retinal vessel structure and function, HRV time-and-frequency domain parameters during FLIP and cTnT to provide further evidence in support of the brain–retina–heart link. Due to the increased SNS tone, well documented in the SABPA African group [9, 14,15,16,17], we hypothesised that FLIP-induced changes in HRV, compatible with increased SNS activity and tone and/or vagal withdrawal, will relate to altered retinal dynamics and higher levels of cTnT, indicating cardiac stress, specifically in Africans.
Materials and methods
Study design and participants
For the first wave, all teachers enrolled in 43 schools (N = 2170) of the Dr Kenneth Kaunda Education District, North-West Province, South Africa, were invited to participate (Fig. 1). Power analyses were performed for the Sympathetic Activity and Ambulatory Blood Pressure in Africans (SABPA) study cohort by using previous studies for autonomic dysfunction to obtain relevant effect sizes based on differences in biological profiles. Resulting sample sizes of 50–416 enabled explanation of biological differences with a statistical power of 0.8, and a level of significance of 0.05. The target population included urban-dwelling well-educated Black (African) and White African (Caucasian) male and female teachers. This exclusive selection of teachers ensured a socio-economic-education equated sample from a similar working environment; cultural differences could not be excluded. All volunteering teachers had medical aid benefits and were screened to meet study eligibility criteria during the recruitment phase (Fig. 1). Those complying formed the respondent group of 409, but those not complying formed the non-respondent group (N = 62) [18]. Data are currently available for 409 teachers of phase 1 and all were invited to partake in phase 2 from which 359 were followed up in phase 2. The participants voluntarily took part in the study and were initially recruited as part of phase 1 of the SABPA study. All participants included in phase 1 (2008–2009) were invited to take part in phase 2 (2011–2012). Of the initial 409 participants, 359 reported for the second phase of the study. Only participants who took part in the second phase of the study were included for this study as no retinal measurements were taken during the first phase. The study sample comprised of urban male and female African and Caucasian teachers (N = 359) from the North-West Province, Dr Kenneth Kaunda Education District, South Africa, aged 23–68 years. This cohort and selection is fully described elsewhere [18]. All teachers from different schools were informed about the aims of the study and written informed consent was obtained before their inclusion in the study. As they were volunteers, any participant was free to withdraw at any stage of the investigation. Specific exclusion criteria for phase 1 of the SABPA study were however: alpha/beta blocker users, any psychotropic drug (e.g. antidepressants), pregnant or lactating women, blood donation or vaccination 3 months prior to the investigation and tympanum temperatures of greater that 37.5 °C.
Additional exclusion criteria for the current study were epilepsy (N = 1), arrhythmia (N = 17), unusable or poor retinal vessel recordings (N = 41) and unusable HRV data during FLIP (N = 36). The majority of unusable retinal measurements were found in the African group where poor images were obtained of participants who did not want to continue with the measures or could not remain in a sitting position without moving. Regarding retinal vessel reactivity measurements in response to FLIP, only individuals with a quality score of equal or greater than 2.5 were included (Supplementary methods). The majority of the unusable retinal measurements were found in the African group. Finally, a total of 127 Africans and 137 Caucasians were included in the current study (sub-population N = 264) (Fig. 1).
Ethical considerations
The SABPA study obtained ethical approval from the Health Research Ethics Committee of the North-West University (NWU-000-360-7-S6). Written informed consent was obtained from all volunteers prior to participation. All procedures and objectives were explained to the participants prior to their recruitment, and adhered to the institutional guidelines, as stated by the Declaration of Helsinki (2004).
General procedure of investigation
Clinical assessments were done over a 2-day period during the working week. Before 08h00 of the first clinical assessment day, four participating teachers were each fitted with an ambulatory blood pressure measurement (ABPM) and 2-lead ECG monitor device (Cardiotens CE120®; Meditech CE120; Meditech, Budapest, Hungary). A 24 h standardised diet commenced and participants subsequently continued with their normal daily activities, reporting any peculiarities such as nausea, headaches, visual disturbances, palpitations, fainting, stress and physical activity, on the issued 24 h diary cards. At 15h00, participants were transported to the NWU Metabolic Unit Research Facility for clinical measurements including the retinal vessel imaging. The Cardiotens® obtained HRV during retinal vessel analyses as well as event BP prior and after retinal vessel assessments.
Retinal vessel analyses
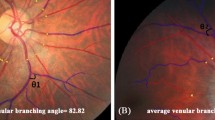
Participants abstained from caffeinated and alcoholic beverages, smoking, strenuous physical activity and food consumption for at least 1 h and no more than 5 h prior to the measurements. They were familiarised with the experimental setup and were examined for acute angle anterior chamber glaucoma risk with a pen light from the side, by a registered nurse. Fifteen minutes prior to the measurement, a drop of Tropicamide (1% Alcon 1% tropicamide and 0.01% benzalkonium chloride (m/v)) induced mydriasis in the right eye. If the right eye was not suitable, the left eye was used. The retinal vessel analyser (Imedos Systems UG, Jena, Germany) was used for digital fundus imaging, with a Carl Zeiss FF450Plus camera (Carl Zeiss, Meditech Jena, Germany), to perform dynamic and static retinal vessel analyses.
For dynamic vessel analysis a standard flicker protocol by IMEDOS Systems was used. During FLIP, the duration of the baseline was 50 s, followed by a 20 s flicker period and an 80 s recovery (also referred to as the second baseline) period. There were three flicker cycles in total, lasting an added total of 350 s for the entire measurement. The camera was set at a 30° angle with the participant focusing on the tip of a fixation rod, and one arteriole and one venular segment (as long as possible) were primarily selected in the upper or lower temporal quadrant of the fundus image. The quality of the FLIP measurements for each participant were assessed subjectively using a newly developed, previously described, scoring method and extensively described by Kotliar et al. [19] and in the Supplementary Methods.
Absolute vessel diameters (measured in standardised measuring units (MU)) were determined for each measurement. Each were calculated individually as the median value over the last 30 s of the first baseline phase prior to FLIP. Parameters derived from the smoothed averaged curve during FLIP, used in the current study, included: (1) The percentage maximal dilation in response to FLIP (MD), and (2) The percentage absolute maximal constriction after FLIP (MC) was the minimum value occurring after maximum FLIP induced dilation and expressed as a percentage from baseline.
Dynamic vessel analysis with flicker stimulation was performed first, followed by image capturing for static vessel analysis. In static vessel analysis we calculated the central retinal artery and vein equivalent (CRAE and CRVE, respectively) and subsequently determined the arteriovenous ratio (AVR) as previously described in the SABPA-study [17]. The presence of retinopathy and AV-nicking was determined by a registered ophthalmologist from a colour retinal image (Supplementary Methods).
Heart rate variability calculations during FLIP
HRV measures, time-and frequency domain analyses, were obtained with the Cardiotens® (Meditech CE120®; Meditech, Budapest, Hungary) and calculated using the CardioVisions® (Meditech CE120®; Meditech, Budapest, Hungary) software. The FLIP response window was viewed for each participant and a minimum window of 15 min was selected. This window was selected to include FLIP and post-FLIP analyses and is referred to as dynamic vessel analyses (DVA)-FLIP. The software removed all arrhythmias and extra-ventricular beats and additional outliers were manually removed before frequency and time domain variables were calculated for the selected window (please refer to Fig. S1). Variables used included time domain parameters, standard deviation of the NN intervals (SDNN), the root-mean squared of the standard deviations of successive RR-intervals (rMSSD) as well as the triangular index (HRVti). The HRVti is an index of the pulse variability based on a triangular interpolation method in the given time interval. The histogram assesses the relationship between the total number of RR intervals detected and the RR interval variation. The triangular HRV index considers the major peak of the histogram as a triangle with its baseline width corresponding to the amount of RR interval variability, its height corresponds to the most frequently observed duration of RR intervals and its area corresponds to the total number of all RR intervals used to construct it (Fig. S2b). NN-intervals refer to the intervals between normal R–R peaks. During a measurement, artefacts may arise due to arrhythmic events or sensor errors [5]. This may lead to abnormal R–R peaks, which may lead to incorrect statistical calculations. To ensure reliability and validity of data, only normal R–R peaks are selected. Please refer to the methods diagram on how the HRV parameters were calculated (Fig. S1). In practice, however, RR-intervals and NN-intervals are synonymous. The use of ‘NN-intervals' is to emphasise that the normal R–R peaks were used. Frequency domain parameters included the normalised units (nu) of the low-frequency bands (LFnu), high-frequency bands (HFnu) and the LF/HF ratio (Fig. S2a, b). Frequency domain analyses, over shorter periods of time, are more often employed as parameters as these are better equipped to discriminate between the contributions of SNS and parasympathetic (PNS), as they manifest in two overlapping frequency bands. The time domain parameters selected (specifically SDNN and rMSSD) are also used to investigate recordings of short durations [5, 20]. It is important to note that components of HRV provide measurement of the degree of autonomic modulations rather than the level of ANS tone [5]. Therefore, for the sake of clarity, all references to time domain parameters will be described in terms of SNS activity and references to frequency domain parameters will indicate SNS modulation. Please refer to Table S1a, S1b for an overview on each HRV parameter.
Biochemical analyses
Fasting blood samples were obtained from the antebrachial vein branches of each participant’s dominant arm with a sterile winged infusion set, by a registered nurse. Blood samples were handled according to the standardised protocol and all samples were frozen at −80 °C until analysed in duplicate. cTnT was measured via an electrochemiluminescence method on the Roche® e411 (Roche®, Basel, Switzerland). Inter-batch variability 4.6%; intra-batch variability 4.2%. Below detectable limit cTnT values (31.3% of all cTnT analyses, N = 104) were logarithmically calculated according to a previously developed method [21].
Statistical analyses
Statistica version 13.3 (TIBCO Software Inc., Palo Alto, USA, 2018) was used for data analyses. Normality of all variables was tested within ethnic groups. Logarithmically transformed γGT, cotinine and HbA1c levels were used in multivariable correlation models. Characteristics between ethnic groups were calculated with t tests. Chi-square (X2) statistics were used to analyse proportions and prevalence data. A priori defined covariates included age, body surface area (BSA), cotinine, γGT, HbA1c, total cholesterol/HDL cholesterol ratio, nocturnal dipping status and 24 h pulse pressure [17]. Single two-way ANCOVAs determined ethnic × gender differences for all HRV and retinal vessel markers, independent of a priori selected covariates. One-way ANCOVA was used to determine the least square mean difference in response markers between ethnic groups, independent of a priori selected covariates. Multivariate linear regression analyses were used to determine associations between DVA parameters and HRV markers in several models. The dependent variables included (1) structure: CRAE, CRVE and AVR, and (2) reactivity/functionality during FLIP: arteriole maximum constriction, maximum dilation and venular maximum dilation. Independent variables included DVA-FLIP HRV variables SDNN, rMSSD, HRVti, LF, HF, LF/HF ratio and cTnT along with the a priori selected covariates in all models. All HRV parameters were calculated in separate models to avoid co-linearity and baseline HR was adjusted for. Additional adjustment was made for CRVE in the CRAE models and vice versa. For all the aforementioned analyses, significance was set at p < 0.05 (two-tailed) and the F to enter was fixed at 2.5 in regression models.
Sensitivity analyses
Forward stepwise regression analyses, with the same set of covariates, were repeated in several models in both ethnic groups. Excluding participants with diabetes (N = 78), on diabetic treatment (N = 29) and those using any form of hypertensive treatment (N = 76), did not influence the outcome.
Results
We applied a hypothesis-driven approach where higher sympathetic activation [22, 23] and stroke risk [13] were shown in the SABPA Africans. Hence we commenced with the current investigation to determine ethnic differences for HRV responses to FLIP; interaction testing revealed significant differences between Africans and Caucasians, but not between gender groups, independent of a priori selected covariates for CRVE (F1, 254 = 8.0, p = 0.003); Arteriole maximal dilation (%) (F1, 240 = 19.27, p < 0.001); Venular maximal dilation (%) (F1, 240 = 20.65, p = 0.024); cTnT (F1, 254 = 27.1, p < 0.001); FLIP rMSSD (F1, 236 = 17.23, p < 0.001); and FLIP HRVti (F1, 236 = 5.30, p = 0.003).
Baseline characteristics
Caucasians were older, Africans had lower BSA, but a poorer cardio-metabolic profile with higher CRP, HbA1c and total cholesterol: HDL cholesterol levels compared to Caucasians (p < 0.05). Similar cTnT values were observed between ethnic groups. Africans had higher 24 h BP and PP, pre-and-post FLIP BP (p < 0.001), IOP (p < 0.001) and DOPP (p = 0.010) values. Africans also showed a greater decrease in 24 h HRV in both time-and frequency domain parameters, than their Caucasian counterparts. Hypertensive/diabetic retinopathy (83% vs. 42%) and AV-nicking (77% vs. 26%) were more prevalent in Africans than in Caucasians (Table 1). The baseline characteristics of the SABPA population at phase 1 is shown in Table S2.
Adjusted retinal calibres and FLIP HRV parameters
In Table 2, the Africans revealed wider venules (p < 0.001) and a smaller ARV (p < 0.001). A comparison between dynamic retinal vessel parameters revealed that both arteriolar and venular maximum dilation in response to FLIP were greater in Africans (p < 0.05). Lower SDNN, rMSSD and HRVti values during FLIP were evident in the African group (p = 0.004). Frequency domain parameters during FLIP did not differ significantly between ethnicities. Overall Africans presented with greater decreases in 24 h and DVA-FLIP HRV than Caucasians (Fig. 2). In addtion, systemic cTnT levels did not significantly differ between Africans and Caucasians (Table S3).
HRV, dynamic retinal calibres and SNS activity
During FLIP (Table 3.1), arteriolar maximum dilation inversely associated with rMSSD (p = 0.030) in Africans. Again in this group, arteriolar maximum constriction inversely associated with both LFnu (p = 0.003) and HFnu (p = 0.021). Venular maximum dilation inversely associated with SDNN and rMSSD, respectively, but positively with LF/HF (p < 0.01) in Africans. However, in Caucasians, arteriolar maximum dilation positively associated with LFnu (p = 0.004) and arteriolar maximum constriction positively associated with HRVti (p = 0.015). Systemic cTnT levels were inversely associated with rMSSD (p = 0.040) in Africans only (Table S4).
HRV, static retinal calibres and SNS activity
In (Table 3.2), inverse associations emerged for CRVE with SDNN and LFnu (p = 0.009), as well as for AVR with rMSSD and HFnu (p = 0.027), in Africans exclusively. In Caucasians there were positive associations of CRAE with HFnu, LF/HF, SDNN, rMSSD and HRVti (p < 0.05). In contrast CRAE and AVR showed inverse associations with LFnu (p = 0.020). In the same group, AVR was positively associated with HFnu, LF/HF, SDNN, rMSSD and HRVti (p < 0.05).
Discussion
To our knowledge, this is the first study to link retinal structure and function with HRV parameters measured during DVA-FLIP. We investigated the relationship between retinal vessel structure and function, HRV parameters during DVA-FLIP analyses as well as the cardiac stress marker, cTnT, in a bi-ethnic cohort from South Africa. In Africans, attenuated arteriolar constriction and venular dilation to FLIP associated with decreased DVA-FLIP HRV (frequency-and-time domain measures). Higher systemic levels of cTnT were also associated with decreased HRV during FLIP. Findings support that dysregulation of the ANS may be associated with altered haemodynamics of the retinal and possibly the cerebral microvasculature. This may also imply that disrupted retinal (and cerebral) autoregulation may be a general result of systemic ANS dysfunction, indicating the importance of central control by the brain on microcirculatory systems in different vascular beds.
HRV, dynamic retinal calibres and SNS activity
Generally, myogenic, neurogenic and humoral processes are assumed to be involved in autoregulation of retinal vascular function [24]. In addition, studies have indicated that retinal microvasculature reaction to increased demands depends on predisposing factors such as stress susceptibility, endothelial function, circulating neuroendocrine, cardiac peptides and inflammatory factors [25]. Several studies have shown that the SABPA-Africans present with an altered stress responsivity, mainly related to SNS hyperactivity [9, 15, 17]. In the same cohort, such SNS hyperactivity associated with alterations in microvascular neural nitric oxide (NO) responses [14] and increased norepinephrine levels [17]. SNS hyperactivity is one of the main driving forces of hypertension [7]. The current findings support this notion as Africans presented with higher 24 h BP, pre-and-post FLIP BP, as well as higher pulse pressure. Such high-pressure conditions entail inadequate perfusion of peripheral tissues, but also diminished myogenic controlled mechanisms, defined as the micro-vessels’ autoregulatory capacity [26, 27]. Autoregulation of the retinal microvasculature is apparently mainly mediated by responses to NO [28]. Interestingly, although retinal autoregulation was impaired in diabetic patients, retinal responses to exogenous NO were similar to that of healthy controls [29], implying maintained endothelium sensitivity to NO in diabetes. Therefore, other factors, including ANS dysregulation and SNS hyperactivity, common in patients with diabetes, might play an important role in altered vasoactivity of retinal vessels.
Indeed, 37% of the African group were non-dippers—which in itself is an established feature of a sustained increase in SNS tone. Furthermore, 83% of the African cohort exhibited retinopathy that is prevalent in hypertension and diabetes, both of which relate to systemic autonomic neuropathy and increased SNS tone [30, 31]. Decreases in 24 h ambulatory time (SDNN, rMSSD and HRVti) and frequency (LFnu, HFnu and LF/HF ratio) domain parameters were evident in Africans compared to Caucasians. Reduced HRV indicates an increase in SNS activity and modulation and/or decreased vagal modulation [5]. A hyperactive SNS may compromise peripheral tissue perfusion, but also retinal and whole brain perfusion [4, 8]. This indicates that FLIP may elicit ANS and particularly SNS-driven responses of the retinal vasculature and its autoregulative capacity.
In Africans, attenuated retinal arteriolar as well as venular dilatory responses are associated with decreased DVA-FLIP HRV time-and frequency domain parameters. Arteriolar and venular dilation inversely associated with markers of PNS activity (rMSSD) and positively with increased SNS modulation (LFnu). This implies initial vagal withdrawal, possibly followed by increased SNS activity during DVA-FLIP. Long-term lower rMSSD is indicative of decreased vagal tone and has been linked to mental stress [5]. Such attenuated responses may reflect cerebral vasculature responses, and recent investigations have shown that vagal withdrawal relates to poorer whole brain perfusion [4, 8]. Arteriolar constriction was inversely associated with both LFnu and HFnu, indicating a decreased power in all spectral bands, typically observed during and attributed to an insufficient SNS response [5]. Indeed, β-adrenergic hypo-responsivity preceded by increased SNS activity were reported in SABPA-Africans [17]. In Africans, as systemic autonomic dysfunction worsens, the ability of the retinal vasculature to auto-regulate decreases. Preliminary histological evidence suggested the presence of alpha-adrenergic receptors in the mammalian retinal vessels [32], and the retinal vessels also contain catecholaminergic amacrine cells. Both infer that the ANS may directly influence the contractile abilities of the retinal microvasculature. Irrespective of these mechanisms, systemic haemodynamics may alter autoregulation capacity, depending on the degree of hemodynamic change that occurs prior to the microvasculature bed (Eularian conservation of fluid) [33]. Therefore, generalised SNS hyperactivity may elicit loss of tone and/or altered haemodynamics of the retinal vasculature. This also implies that if long-term SNS hyperactivity is present, impeded autoregulation should persist even after the stressor (FLIP) has ceased.
HRV, static retinal calibres and SNS activity
In Africans, wider venules and a smaller ARV were positively associated with increased SNS modulation (LFnu) and diminished vagal modulation (HFnu). This introduces the possibility that the FLIP-induced stress response may persist due to the presence of general SNS hyperactivity. Therefore, sustained systemic SNS hyperactivity may govern a prolonged response to a stressor. SNS hyperactivity further encroaches on the retinal vasculature’s autoregulatory capacities. In contrast, in Caucasians, post-FLIP static retinal analyses (arteriolar calibres and AVR) associated with both time-and frequency domain parameters are indicative of a restored ANS balance. Such maintained sympatho-vagal balance is characterised by an increase in vagal modulation (HFnu) and decreased SNS modulation (LFnu) [5]. Our results support the notion that the SNS exerts a significant effect on smooth muscle tone of the retinal vasculature, either directly or indirectly.
HRV during FLIP, cTnT and SNS activity—the brain–retina–heart link
We previously proposed a link between increased cTnT and attenuated retinal vasculature function [11]. Current findings support this, specifically within the context of cTnT’s association with SNS function and FLIP as a mental/physical stressor. The inverse association between systemic cTnT and rMSSD during FLIP in Africans provides further support for the brain–heart link [33,34,35]. Higher levels of cTnT, as well as mental stress-induced increases in cTnT were related to SNS hyperactivity in Africans [9]. Furthermore, additional cTnT release was linked to increased activity of the midcingulate and subgenual anterior cingulate, located in the prefrontal cortex and identified as a primary seat of SNS activity [8]. This supports our observation of an association between cTnT and attenuated retinal arteriolar responses. The latter might link systemic levels of cTnT with vagal withdrawal and increased SNS tone.
Although speculative, we suggest that in the presence of pre-existing SNS hyperactivity, additional SNS activity provoked during FLIP further increases SNS modulation, thereby blood flow and activity in areas of the cingulate gyrus, possibly resulting in additional systemic cTnT release. Activation of the amygdala and the hippocampus also occur, and this cascade of activation finally launches a systemic response via input from the hypothalamus [7] and/or thalamus [36]. During FLIP, an already hyperactive SNS will further be challenged, thereby causing further reduction in HRV indicating greater SNS activity and modulation, in an attempt to maintain adequate perfusion. Existing pressure-induced endothelial dysfunction and impaired smooth muscle responses will manifest as attenuated retinal vessel dilatory and constriction responses. This increased SNS activity/modulation may lead to a prolonged vessel response, even after the stressor has ceased, indicating difficulty in re-establishing proper ANS balance. As a consequence the autoregulatory capacity of the retinal microvasculature will be impaired (Fig. 3).
Translational relevance—from Cardiology to Ophthalmology
SNS hyperactivity may cause loss of tone and/or altered haemodynamics of the retinal microvasculature. Attenuated arteriolar and venular responses during FLIP, accompanied by decreased HRV variables, imply that the SNS exerts a significant effect on the smooth muscle tone of the retinal vasculature, either directly or indirectly. Indeed, chemical stimulation of autonomic regulatory neurons in the dorsomedial and perifornical nuclei in the hypothalamus showed marked increases in IOP [37]. Agnifili et al. [38] suggested that IOP variation was greater in patients suffering from primary open-angle glaucoma (POAG) compared to normal controls. Such fluctuations in IOP, together with altered haemodynamics and retinal blood-flow regulation, may possibly contribute to the pathogenesis of and glaucomatous damage in POAG [39]. The SABPA-Africans with higher IOP and altered haemodynamics might therefore have a greater susceptibility for glaucomatous damage. Indeed, defective perfusion played a role in the appearance and exacerbation of glaucomatous optic neuropathy [37, 39]. Sympathetic dominance and depressed HRV were also documented in patients with normal-tension glaucoma [37]. In addition, associated vascular dysregulation was linked to SNS hyperactivity, manifested as blunted BP responses and decreased optic nerve head blood flow in response to cold provocation [40]. This may indicate that the SABPA-African cohort may have a greater risk for optic nerve damage.
Limitations and recommendations
Limitations of the current study may include the specific population (only including African and Caucasians teachers from one demographic area) as well as the cross-sectional design, which prevents identification of physiological mechanistic cause-and-effect relationships. Several investigations have identified increased psychosocial stress experienced by this vulnerable African cohort, as one of the main contributors to this observed higher risk profile. However, the exploration of psycho-social stress influences are not within the scope of the current investigation and we suggest assessing the effect of psychosocial stress on the structure and function of the retinal vasculature, cardiac stress markers and stroke risk. We further recommend future retinal vessel investigations to include beat-to-beat BP monitoring, as cardio-metabolic demands increase during the FLIP, and blood pressure variations will occur in order to maintain homeostasis.
Summary
What was known before
-
It is largely accepted that the ANS does not exert a significant effect on the retinal microvasculature’s autoregulatory capabilities. Yet, mean retinal vessel responses to systemic sympathetic stimulation were significantly reduced in sympathectomised eyes. However, no study to date has examined the time-and frequency domain measures of HRV during flicker light stimulation, and related these measures to the dynamic changes in the retinal vasculature.
What this study adds
-
This is the first study to directly link retinal structure and function with HRV parameters measured during flicker light, as well as cardiac stress. Findings support that SNS hyperactivity may cause loss of tone and/or altered hemodynamics of the retinal microvasculature. Attenuated arteriolar and venular responses during flicker light, accompanied by decreased HRV variables, imply that the SNS exerts a significant effect on the smooth muscle tone of the retinal vasculature. Disrupted retinal autoregulation may be a result of general, sustained ANS dysfunction, exemplifying central control by the brain on all systemic regulatory functions, regardless of the vascular bed. SNS hyperactivity may also increase the risk for glaucotamous damage and optic nerve damage, specifically in Africans.
Change history
04 September 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41433-019-0570-4
References
Huemer KH, Garhöfer G, Zawinka C, Golestani E, Litschauer B, Schmetterer L, et al. Effects of dopamine on human retinal vessel diameters and its modulation during flicker stimulation. Am J Physiol Heart Circ Physiol. 2003;284:358–63.
Lanigan LP, Clark CV, Hill DW. Retinal circulation responses to systemic autonomic nerve stimulation. Eye. 1988;2:412–7.
Lanigan LP, Birche R, Clark CV, Hill DW. The effect of cervical sympathectomy on retinal vessel responses to systemic autonomic stimulation. Eye. 1990;4:181–9.
Pemp B, Palkovits S, Howorka K, Pumprla J, Sacu S, Garhöfer G, et al. Correlation of retinal neurodegeneration with measures of peripheral autonomic neuropathy in type 1 diabetes. Acta Opthalmol. 2018. https://doi.org/10.1111/aos.13733.
Malik M, Bigger T, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Task force of the European Society of Cariology and the North American Society of pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 2018;93:1043–65.
Amiya E, Watanabe M, Komuro I. The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 2014;7:109–19.
Seravalle G, Mancia G, Grassi G. The sympathetic nervous sytem in hypertension. In: Landsberg L, editor. Pheochromocytomas, paragangliomas and disorders of the sympathoadrenal system. Springer International Publishing AG; 2018. Contemporary Endocrinology. https://doi.org/10.1007/978-3-319-77048-2_14.
Allen B, Jennings RJ, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52:277–87.
Malan L, Hamer M, von Känel R, Lambert GW, Delport R, Steyn HS, et al. Chronic defensiveness and neuroendocrine dysfunction reflect a novel cardiac troponin T cut point: the SABPA study. Psychoneuroendocrinology. 2017;28:30–27.
Reimann M, Hamer M, Schlaich M, Malan NT, Ruedinger H, Ziemssen T, et al. Autonomic responses to stress in Black versus Caucasian Africans: the SABPA study. Psychophysiology. 2012;12:454–61.
Micieli G, Cavallini A. The autonomic nervous system and ischemic stroke: a reciprocal interdependence. Clin Auton Res. 2008;18:308–17.
Reddy K, Khaliq A, Henning RJ. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J Cardiol. 2015;7:243–76.
Wentzel A, Malan L, Smith W, von Känel R, Malan NT. Retinal vasculature reactivity during flicker light provocation, cardiac stress and stroke risk in Africans: the SABPA study. Transl Stroke Res. 2018. https://doi.org/10.1007/s12975-018-0673-4.
Malan L, Hamer M, von Känel R, Schlaich MP, Riemann M, Malan NT, et al. Chronic depression symptoms and salivary NOx are associated with retinal vascular dysregulation: the SABPA study. Biol Chem. 2016;55-56:10–7.
Malan L, Hamer M, Schlaich MP, Lambert GL, Harvey BH, Malan NT, et al. Facilitated defensive coping, silent ischaemia and ECG-left ventricular hypertrophy: the SABPA study. J Hypertens. 2012;30:543–50.
Malan L, Hamer M, Schlaich MP, Lambert GW, Ziemssen T, Malan NT, et al. Defensive coping facilitates higher blood pressure and early sub-clinical structural vascular disease via alterations in heart rate variability: the SABPA study. Atherosclerosis. 2013;227:391–7.
Malan NT, von Känel R, Smith W, Lambert GW, Vilser W, Eikelis N, et al. A challenged sympathetic system is associated with retinal vascular calibre in a black male cohort: the SABPA study. In: Microcirculation Revisited - From Molecules to Clinical Practice, Helena Lenasi. London, United Kingdom: IntechOpen; 2016. https://doi.org/10.5772/63515. Available from: https://www.intechopen.com/books/microcirculation-revisited-from-molecules-toclinical-practice/a-challenged-sympathetic-system-is-associated-with-retinal-vascular-calibre-in-a-black-male-cohort-t
Malan L, Hamer M, Frasure-Smith N, Steyn HS, Malan NT. Cohort profile: sympathetic activity and ambulatory blood pressure in Africans (SABPA) prospective cohort study. Int J Epidemiol. 2015;44:1814–22.
Kotliar K, Hauser C, Ortner M, Muggenthaler C, Diehl-Schmid J, Anger mann S, et al. Altered neurovascular coupling as measured by optical imaging: a biomarker for Alzheimer’s disease. Sci Rep. 2017;7:12906. https://doi.org/10.1038/s41598-017-13349-5.
Von Rosenberg W, Chanwimalueacng T, Adjei T, Jaffer U, Goverdovsky V, Mandic DP. Resolving ambiguities in the LF/HF ratio:LF-HF scatterplots for the categorization of mental and physical stress from HRV. Front Physiol. 2017;8:360. https://doi.org/10.3389/fphys.2017.00360.
Croghan C, Egeghy PP. In: Methods of dealing with values below the limit of detection using SAS. Presented at Southern SAS User Group, St. Petersburg, FL, 28 June 2012. cfpub.epa.gov/ordpubs/nerlpubs/recordisplay.cfm?deid¼62970.
Malan L, Hamer M, von Känel R, Lambert GW, Delport R, Steyn H, et al. Chronic defensiveness and neuroendocrine dysfunction reflect a novel cardiac troponin T cut-point: the SABPA study. Psychoneuroendocrinology. 2017;85:20–7.
Wentzel A, Malan L, von Känel R, Malan NT. Ethnic-specific cardiac troponin T acute mental stress responses reflect ethnic specific RaVL cut-point: the SABPA study. Am J Epidemiol. 2019.
Jandrasits K, Polak K, Luksch A, Stark B, Dorner GT, Eichler HG, et al. Effects of atropine and propranolol on retinal vessel diameters during isometric exercise. Opthalmic Res. 2001;33:185–90.
Ma H, Guo L, Huang D, Wang L, Guo L, Geng Q, et al. The role of myocardial microvasculature in mental stress-induced myocardial ischemia. Clin Cardiol. 2016;39:234–9.
Chang-Ling T. The blood retinal interface: similarities and contrasts with the blood-brain interface. In: Blood-brain barriers. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co KgaA; 2006. p. 701–24.
Terai N, Spoerl E, Pillunat LE, Stodtmeister R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Opthalmol. 2012;90:524–8.
Mandecka A, Dawczynski J, Blum M, Müller N, Kloos C, Wolf G, et al. Influence of flicker light on retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–52.
Lott MEJ, Slocomb JE, Shivkumar V, Smith B, Quillen D, Gabbay RA, et al. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Opthalmol. 2013;91:e462–9.
Lanigan LP, Clark CV, Allawi J, Hill DW, Keen H. Responses of the retinal circulation to systemic autonomic stimulation in diabetes mellitus. Eye. 1989;3:39–47.
Al-Fiahd AH, Farouque O, Kawasaki R, Nguyen TT, Uddin N, Freeman M, et al. Retinal microvascular structure and function in patients with risk factors of atherosclerosis and coronary artery disease. Atherosclerosis. 2014;233:478–84.
Cohen J, Hadjiconstantinou M. Identification of epinephrine and phenylethanolamine-n-methyl transmethyl transferase in rat retina. Fed Proc. 1984;433:2725–28.
Badin G, Crisciani F. Variational formulation of fluid and geophysical fluid dynamics: mechanics, symmetries and conservation laws. Springer; 2018. p. 218. https://doi.org/10.1007/978-3-319-59695-2. ISBN 978-3-319-59694-5.
Lazzarino AI, Hamer M, Gaze D, Collinson P, Rumley A, Lowe G, et al. The association between fibrinogen reactivity to mental stress and high-sensitivity cardiac troponin T in healthy adults. Psychoneuroendocrinology. 2015;59:37–48.
Lazzarino AI, Hamer M, Gaze D, Collinson P, Steptoe A. The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. J Am Coll Cardiol. 2013;62:1694–701.
Beas BS, Wright BJ, Skirzewski M, Leng Y, Hyun JH, Koita O, et al. The locus coeruleus disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci. 2018;21:963–73.
Pasquale LR. Vascular and autonomic dysfunction in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27:94–101.
Agnifili L, Mastropasqua R, Frezzotti P, Fasanella V, Motolese I, Pedrotti E, et al. Circadian intraocular pressure in healthy subjects, primary open-angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015;93:14–21.
Ghergel D, Hosking SL, Orgul S. Autonomic nervous system, circadian rhythms, and primary open-angle glaucoma. Surv Ophthal. 2004;49:492–505.
Grieshaber MC, Mozaffarieh M, Flammer J. What is the link between vascular dysregulation and glaucoma? Surv Ophthal. 2007;52 Suppl 2:S144–54.
Acknowledgements
The present study was partially funded by the National Research Foundation, South African Medical Research Council, ROCHE Diagnostics, North-West University (Potchefstroom Campus), North-West Department of Education South Africa as well as the Metabolic Syndrome institute, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors; therefore, funders do not accept any liability regarding this study.
Supplementary information
Rights and permissions
About this article
Cite this article
Wentzel, A., Malan, L., von Känel, R. et al. Heart rate variability, the dynamic nature of the retinal microvasculature and cardiac stress: providing insight into the brain–retina–heart link: the SABPA study. Eye 34, 835–846 (2020). https://doi.org/10.1038/s41433-019-0515-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-019-0515-y